Deep Vein Arterialization for No-Option Chronic Limb-Threatening Ischemia: An Overview of the LimFlow System and CLariTI Study
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Vascular Disease Management or HMP Global, their employees, and affiliates.
VASCULAR DISEASE MANAGEMENT. 2025;22(9):E64-E69
Abstract
Chronic limb-threatening ischemia (CLTI) is advanced peripheral arterial disease that causes rest pain, ulcers, or gangrene with a high risk of amputation. Affecting an estimated 3.8 million individuals in the United States, CLTI contributes to over 150,000 major amputations annually, particularly among underserved populations. For patients who lack viable options for surgical or endovascular revascularization, treatment options are historically limited. The LimFlow System introduces a novel approach to limb salvage through transcatheter arterialization of the deep venous system. This manuscript outlines the clinical burden of CLTI, explains the LimFlow procedure, and reviews evidence from the CLariTI and PROMISE studies supporting its use in "no-option" patients.
Introduction
Peripheral arterial disease (PAD) affects millions of people worldwide, with chronic limb-threatening ischemia (CLTI) representing its most advanced and debilitating stage. Patients with CLTI experience chronic ischemic rest pain, nonhealing ulcers, and gangrene, often resulting in significant morbidity and mortality. The socioeconomic impact is profound: Major amputation is not only the sixth most expensive surgical procedure in the United States, but also associated with high complication rates, extended hospital stays, readmissions, and frequent loss of independence.1,2
Compounding this challenge, certain demographic groups face significantly higher risks. Black Americans are 4 times more likely to undergo major amputations than their White counterparts. In many cases, patients undergo primary amputation without diagnostic angiography or revascularization attempts.2,3 The pressing need for effective limb salvage strategies in these patients has catalyzed the development of new interventional approaches.
The No-Option CLTI Population
Approximately 20% of patients with CLTI patients fall into the "no-option" category, meaning they have no suitable arterial targets for revascularization due to diffuse or distal disease (Figure 1). These patients typically have a history of multiple failed revascularization procedures or present with anatomical limitations that preclude further surgical or endovascular intervention.4
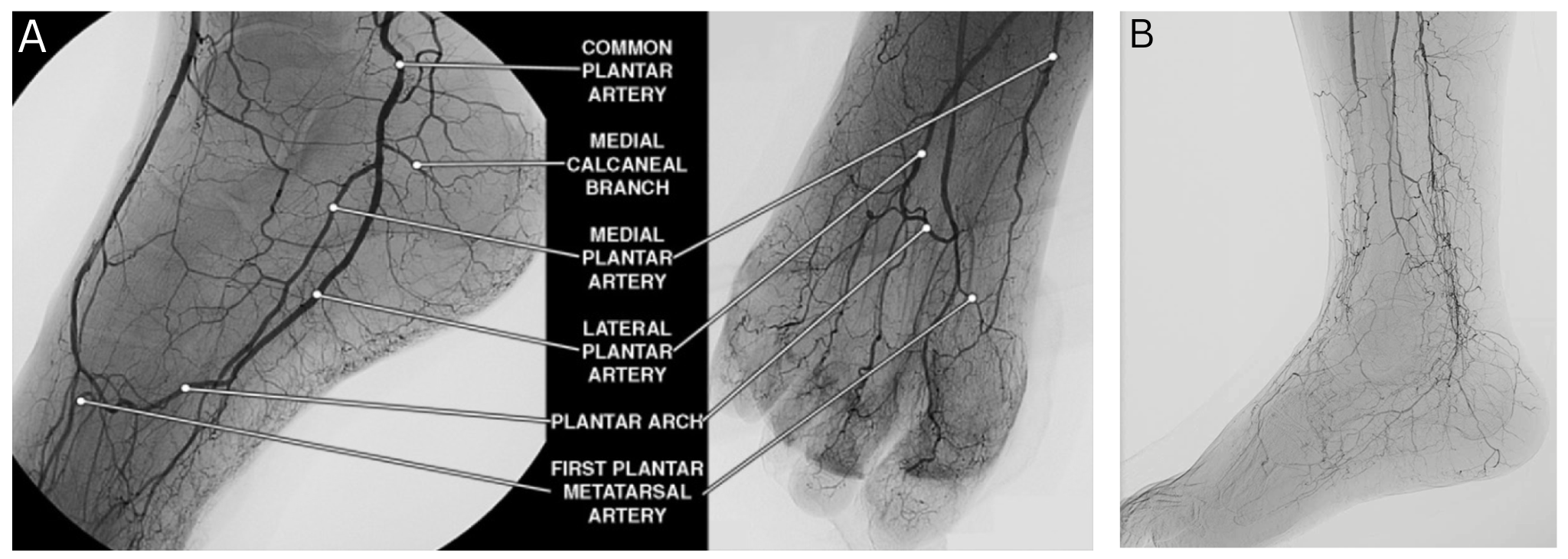
Clinical outcomes in this group are poor. More than half face major amputation or death within 6 months of diagnosis. This grim prognosis underscores the urgency for alternative strategies capable of providing durable limb salvage. Chronic wounds and severely ischemic tissue that cannot be revascularized by standard means typically do not heal, leading to escalating complications and diminished quality of life (Figure 2).
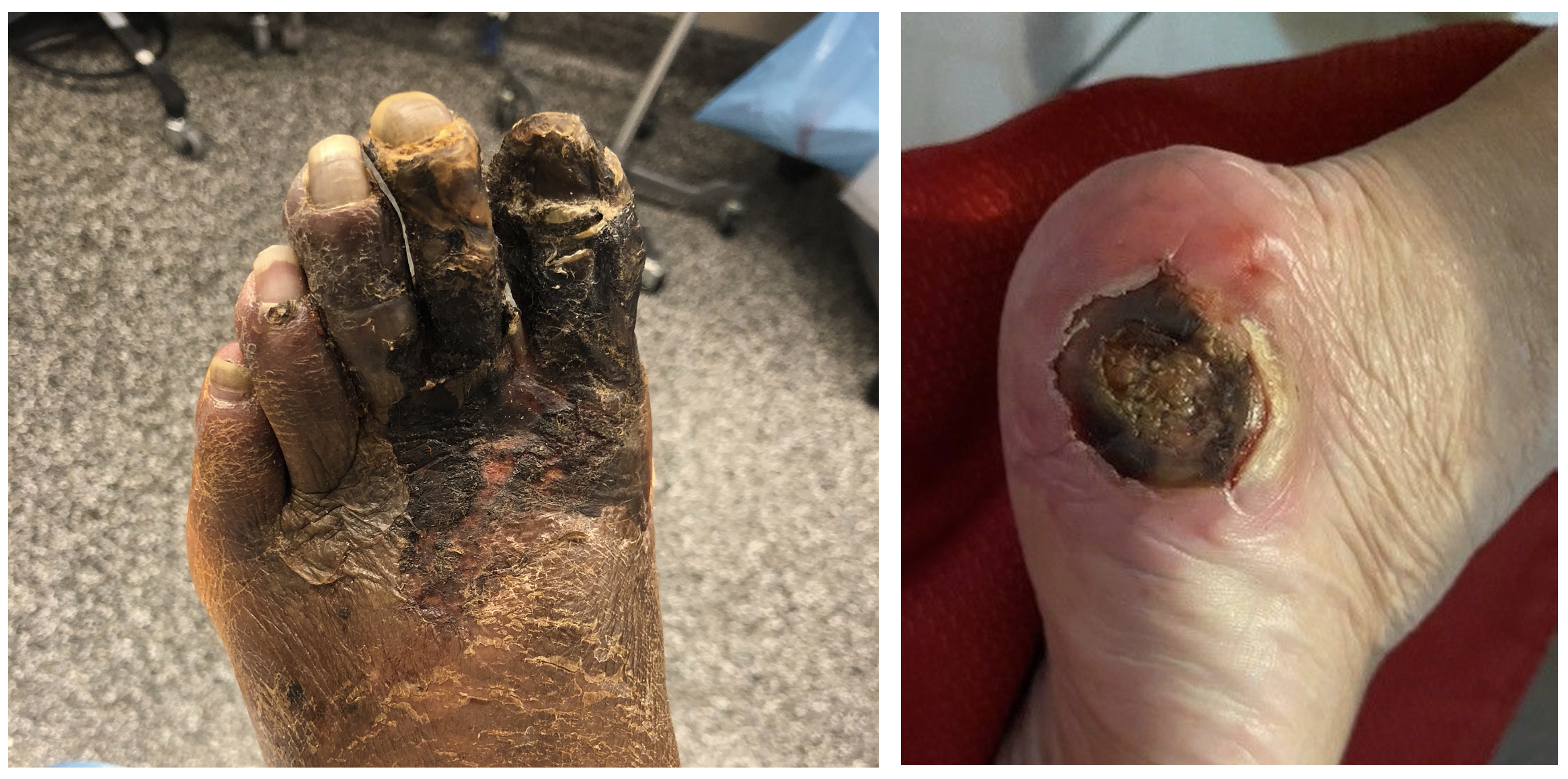
The LimFlow System: Mechanism and Components
The LimFlow System provides an innovative, FDA-approved (2023) endovascular solution specifically indicated for patients with no-option CLTI. The system employs transcatheter arterialization of deep veins, a technique that reroutes arterial blood flow into the venous system to perfuse the ischemic limb (Figure 3).
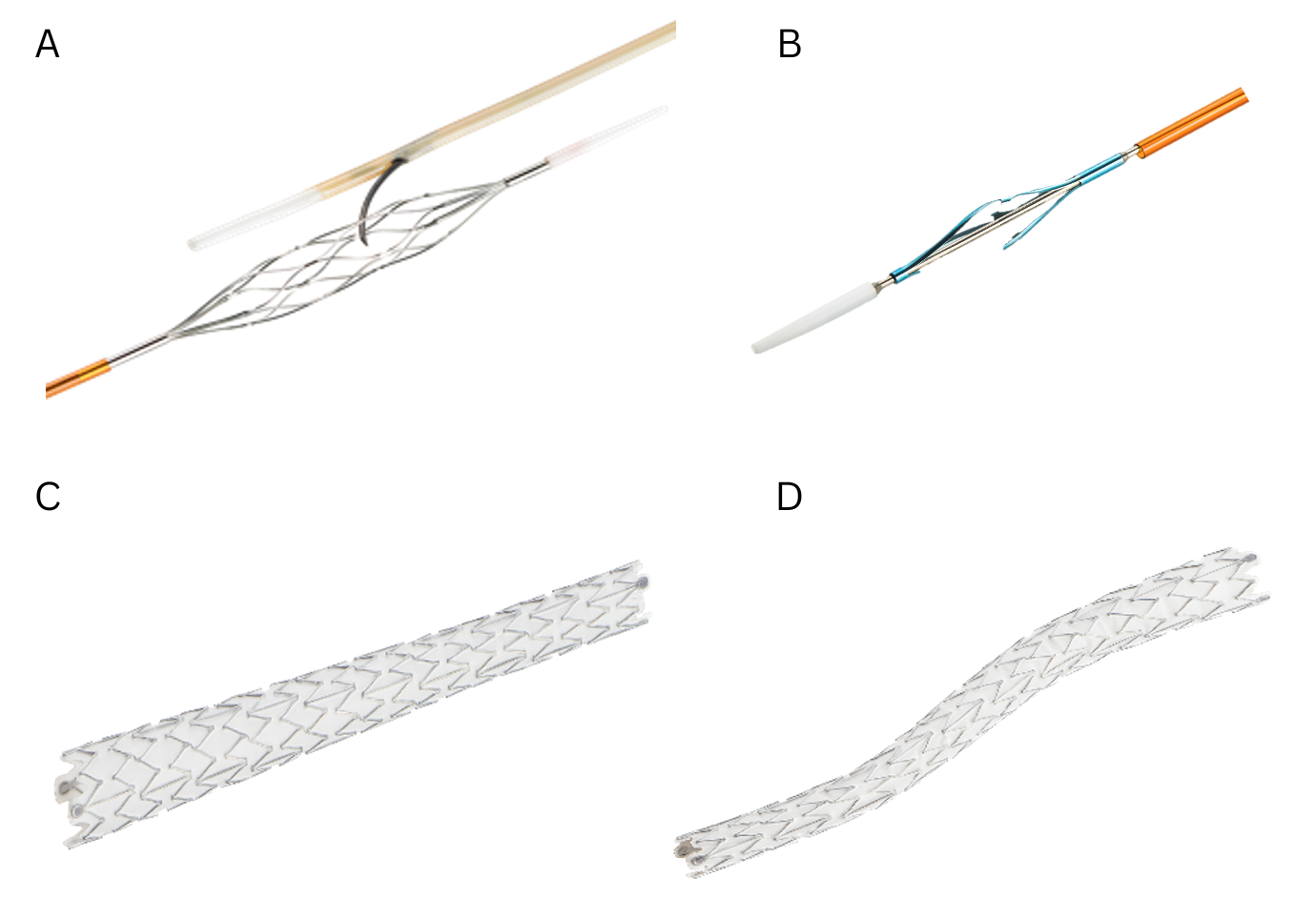
Procedural Steps
1. Arteriovenous crossing: Using proprietary mesh catheters (Figure 3A), a controlled connection is established between the tibial artery and an adjacent deep vein.
2. Vein preparation: A push valvulotome (Figure 3B) is used to render the venous valves incompetent, allowing for retrograde arterial flow.
3. Flow diversion: Conical and straight stent grafts (Figures 3C and 3D) are deployed to direct blood flow through the newly arterialized venous circuit and away from tributaries.
This procedure is designed to be performed in hospital-based settings by specialists in vascular surgery, interventional radiology, or cardiology. The LimFlow System is currently the only device approved for this unique application.
Clinical Evidence: The CLariTI Study
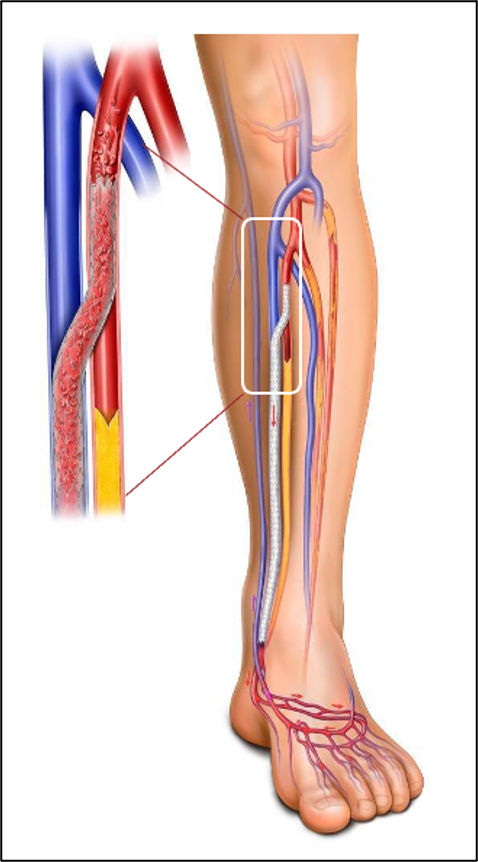
The CLariTI (CLTI Registry to Assess Outcomes and Therapeutic Interventions) study is a prospective, observational registry initiated to evaluate real-world outcomes in patients with no-option CLTI. It provides complementary data to the PROMISE trials by tracking the incidence of amputation, death, and revascularization over a 12-month period.
This single-arm, observational study was conducted at Massachusetts General Hospital. A total of 180 patients were enrolled across 22 sites in the United States. Eligible participants included those with Rutherford Category 5 or 6 CLTI who were considered no-option for further revascularization or had experienced multiple failed revascularization attempts. Patients actively enrolled in the PROMISE II clinical trial were excluded from participation.
Among the no-option cohort (n = 122), the racial and ethnic distribution was diverse: 55% identified as Asian/Caucasian, 36% as Caucasian/Black or African American, and 2% as Asian. A significant portion of the cohort had diabetes, with 71% diagnosed with Type II and 4% with Type I diabetes. Renal disease was also prevalent: 25% had end-stage renal disease, 23% had chronic kidney disease, and 3% had renal insufficiency, while 49% had no documented renal disease.
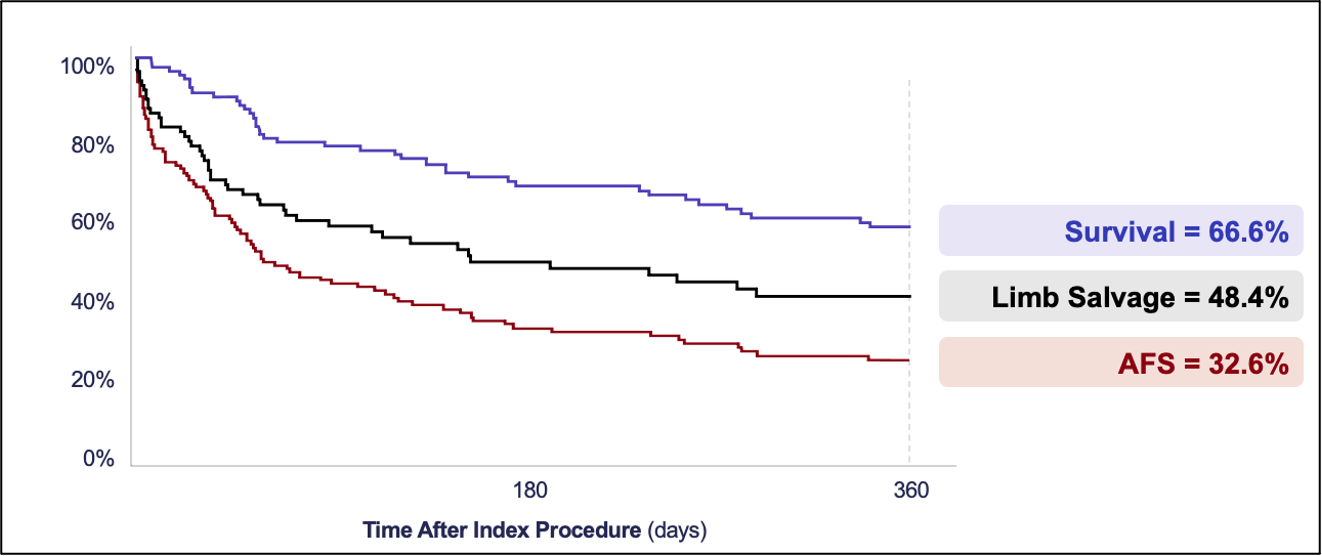
The study observed high amputation-free survival rates and meaningful improvements in wound healing and pain scores over 12 months (Figure 4).5
Long-Term Outcomes: PROMISE Trials
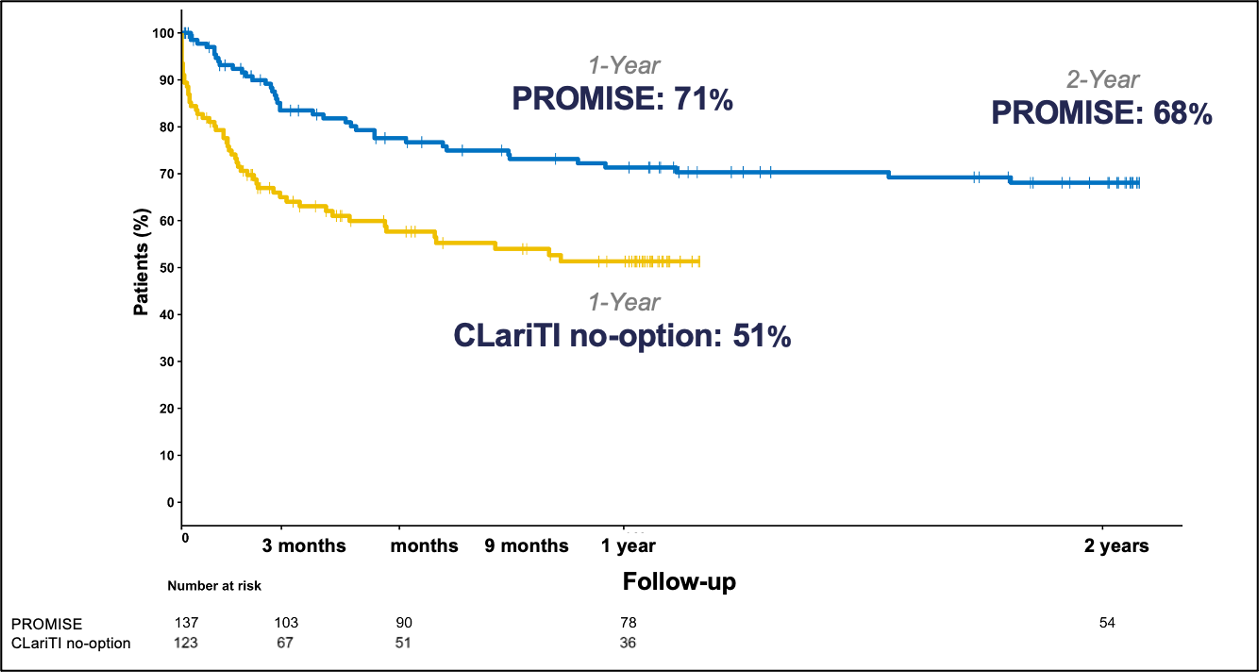
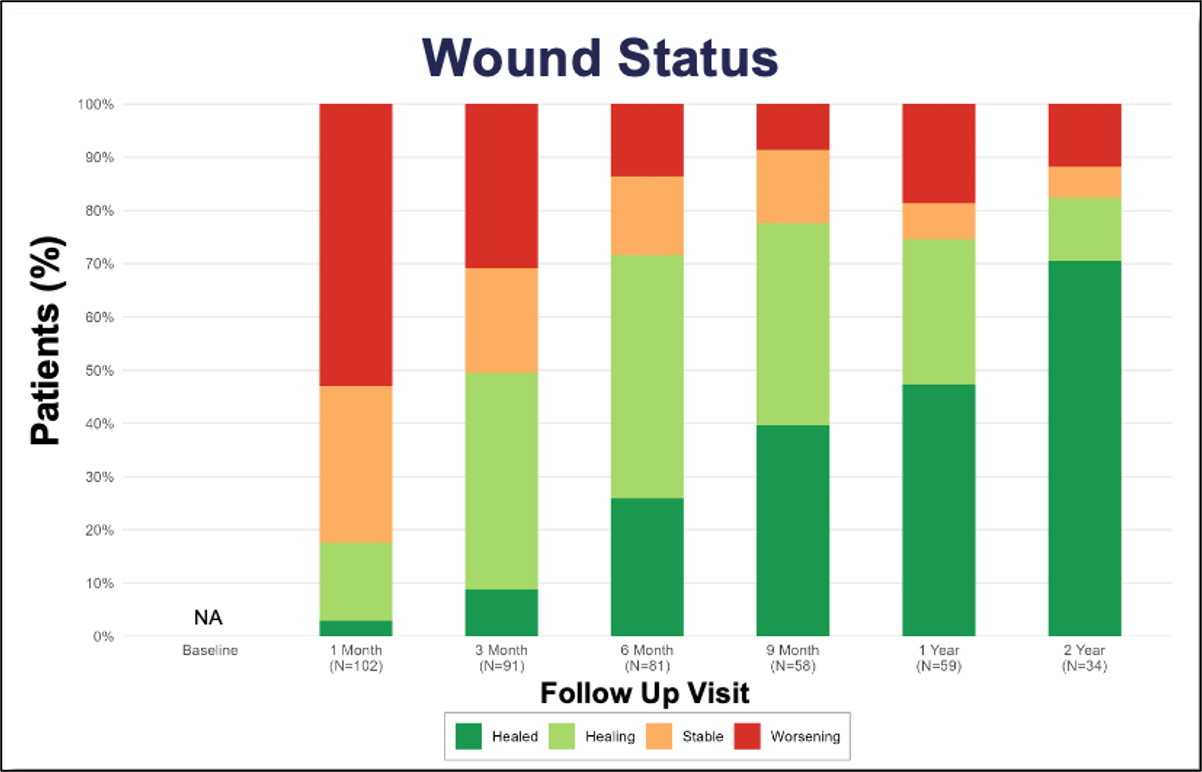
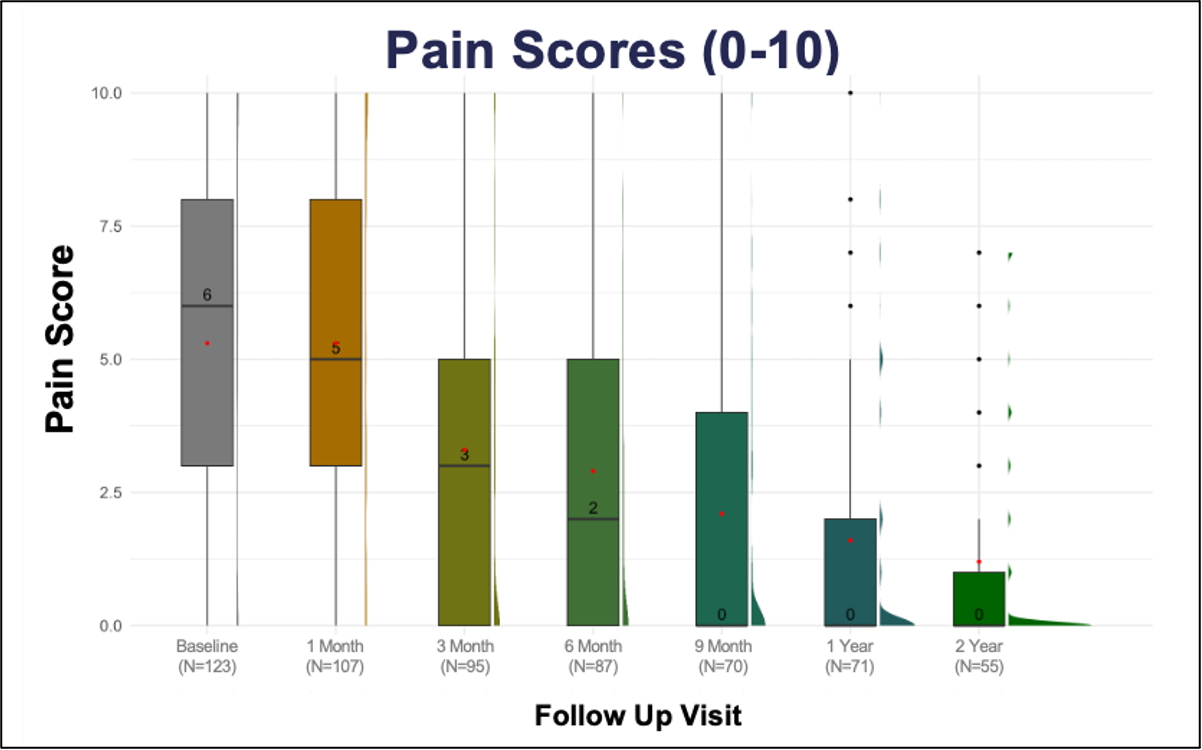
The PROMISE I and II trials evaluated the safety and efficacy of the LimFlow System in a controlled setting, with promising results sustained over 2 years (Figure 5). Data demonstrated high limb salvage rates (Figure 6), significant wound healing (Figure 7), and sustained pain reduction (Figure 8) across multiple follow-up intervals (1, 3, 6, 9, 12, and 24 months).1
Key Metrics:
- Durable limb salvage beyond 24 months
- Continuous improvement in ischemic pain (0-10 pain scale)
- Reduction in nonhealing wound burden
PROMISE III has completed enrollment and is expected to provide further validation and expand on existing data with a more diverse population.
Conclusion
The LimFlow System represents a groundbreaking advancement in the treatment of no-option CLTI, offering hope where traditional approaches have failed. Its FDA approval and inclusion in the 2024 ACC/AHA guidelines affirm its significance in the current vascular treatment landscape. With durable limb salvage, consistent clinical outcomes, and expanding clinical validation, LimFlow establishes a new standard of care for patients previously destined for primary amputation. n
REFERENCES
1. Creager MA, Matsushita K, Arya S, et al. Reducing nontraumatic lower-extremity amputations by 20% by 2030: Time to Get to Our Feet: a policy statement from the American Heart Association. Circulation. 2021;143(17):e875-e891. doi:1.1161/CIR.0000000000000967
2. Yost ML. Cost-benefit analysis of critical limb ischemia in the era of the Affordable Care Act. Endovascular Today. 2014;May:29-36.
3. Gornik HL, Aronow HD, Goodney PP, et al. Correction to: 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS guideline for the management of lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2025;151(14):e918. doi:10.1161/CIR.0000000000001329
4. Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2104;60(3):686–695.e2. doi:10.1016/j.jvs.2014.03.290
5. Powell RJ, Mullin CM, Clair DG, Shishehbor MH, Dua A. Comparison of transcatheter arterialization of deep veins to standard of care in patients with no-option chronic limb threatening ischemia. Ann Vasc Surg. 2024;99:50-57. doi:10.1016/j.avsg.2023.08.010
Affiliations and Disclosures
Darshan Randhawa, MD, Samuel Thomas, and Michael Siah, MD, are from UT Southwestern Medical Center in Dallas, Texas.
Dr Siah is a consultant for LimFlow/Inari. Dr Randhawa and Mr Thomas report no financial relationships or conflicts of interest regarding the content herein.
Manuscript accepted August 11, 2025.
Address for Correspondence: Darshan Randhawa, MD, UT Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas TX 75390. Email: Darshan.Randhawa@UTSouthwestern.edu











