Embolization of Primary and Metastatic Hepatic Tumors Using the Embrace™ Hydrogel Embolic System: Case Studies
Introduction
Embrace™ Hydrogel Embolic System (HES; Instylla) consists of 2 low-viscosity liquid precursors that polymerize intravascularly when injected simultaneously into blood vessels forming a soft, water-based polyethylene glycol hydrogel. Embrace HES is intended to embolize hypervascular tumors in peripheral arteries ≤ 5 mm. The hydrogel has no solvents, does not require sizing to the vessel diameter, and eliminates the possibility of catheter entrapment. The cases herein were part of the prospective, multicenter, randomized Instylla HES Hypervascular Tumor Pivotal Study to evaluate the safety and effectiveness of Embrace HES compared with standard of care transcatheter arterial embolization (TAE) or transcatheter arterial chemoembolization (TACE).
Case 1: Primary Hepatocellular Carcinoma (HCC)
Subject Background: A 69-year-old man was diagnosed with primary HCC confirmed via biopsy, staged BCLC C, and previously underwent chemotherapy treatment (sorafenib). The subject received bland embolization as he was not a surgical candidate nor amenable to Y-90 due to significant lung shunting. The subject subsequently presented with residual 1.5-cm nodular enhancement of a single focal, Segment V lesion.
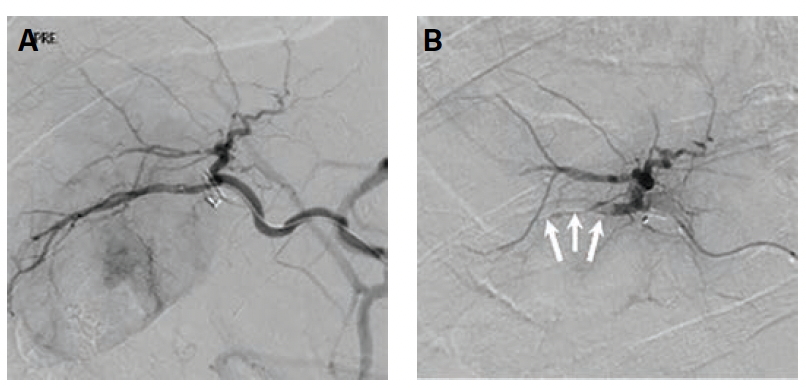
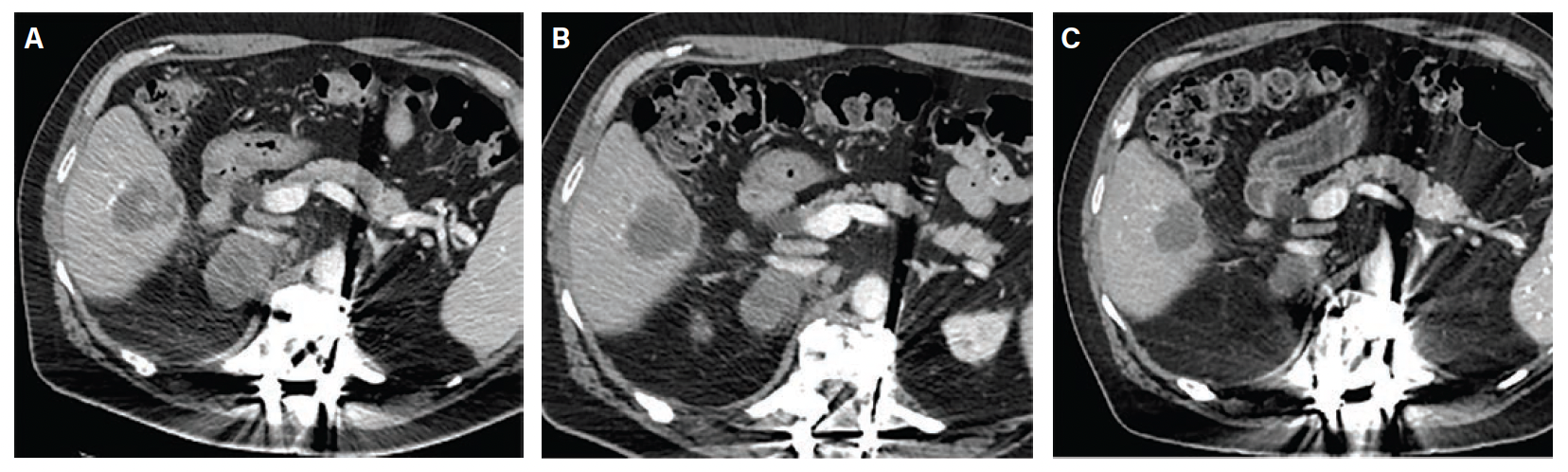
Index Procedure: The subject underwent TAE with Embrace HES under monitored anesthesia care (MAC). Left transradial access was obtained. A coaxial microcatheter system with a 1.7F 162-cm microcatheter (Instylla) inserted in a 2.8F 150-cm microcatheter (Progreat®, Terumo Medical Corporation) was used to embolize a single 3-mm feeder vessel with 1.6 mL of the Embrace (Figure 1). There was no clinical evidence of non-target embolization. Follow-Up: Repeat computed tomography performed at 30- and 90-days post index procedure indicated no evidence of revascularization and no local tumor progression (Figure 2). An independent Core Lab assessed a complete response in accordance with mRECIST criteria at both timepoints.
Case 2: Breast Cancer, Hepatic Metastasis
Subject Background: A 43-year-old woman was diagnosed with breast cancer confirmed via biopsy and underwent chemotherapy treatment including doxorubicin, cyclophosphamide, and taxotere as well as a TACE procedure for a hepatic metastatic lesion. Five years later, the subject presented with a new surgically unresectable, focal encapsulated, Segment V lesion measuring 3.3 cm in diameter.
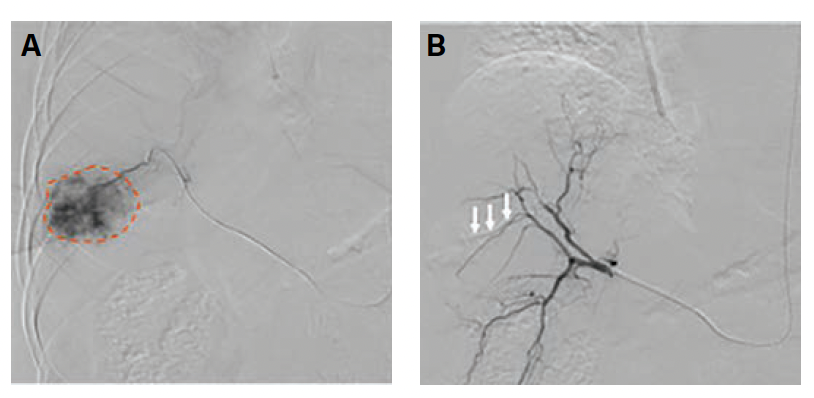
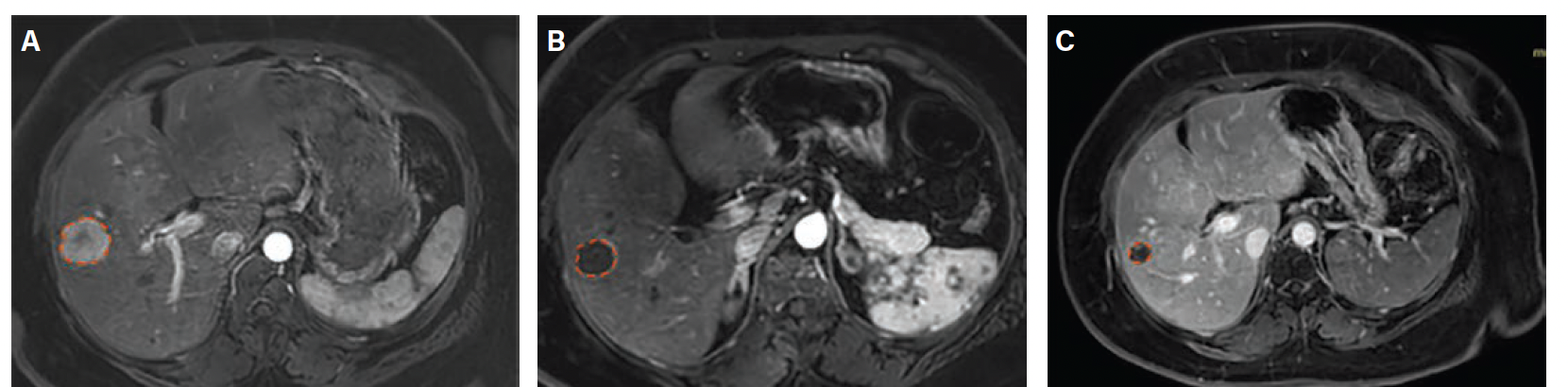
Index Procedure: The subject underwent TAE with Embrace HES under MAC for the new metastatic lesion. Left transradial access was obtained. A coaxial microcatheter system with a 1.7F 162-cm microcatheter (Instylla) inserted in a 2.8F 150-cm microcatheter (Progreat) was used to embolize 3 separate feeder vessels measuring 1.5 mm, 1 mm, and 1.3 mm with a total of 1.9 mL of the Embrace (Figure 3). All 3 vessels were embolized using the same microcatheter system. There was no clinical evidence of non-target embolization. Follow-Up: Repeat magnetic resonance imaging performed at 30- and 90-days post index procedure indicated no evidence of revascularization and no local tumor progression (Figure 4). An independent Core Lab assessed stable disease with a ~28% decrease in tumor diameter at 30 days and partial response with a ~45% decrease in tumor diameter at 90 days in accordance with RECIST 1.1 criteria.
Edgar St. Amour, MD, is from the Central Arkansas Radiation Therapy Institute, Little Rock, Arkansas, and Nadine Abi-Jaoudeh, MD, is from the University of California, Irvine.
Disclosure: Dr. Abi-Jaoudeh reports no financial relationships or conflicts of interest regarding the content herein. Dr. St. Amour has a small equity interest in the form of stock options with Instylla.










