Use of the Penumbra Indigo® System for Femoral Artery Thrombosis
Introduction
The American College of Cardiology, the American Heart Association, and the Society for Cardiovascular Angiography & Interventions do not provide specific guideline recommendations regarding the use of the Penumbra catheter for femoral artery thrombosis. However, the Penumbra® Indigo System is recognized as a viable device for arterial aspiration thrombectomy via a percutaneous approach.
The Society of Interventional Radiology (SIR) identifies percutaneous aspiration thrombectomy as an accepted technique for treating acute lower-extremity arterial occlusions, including iatrogenic femoral artery thrombosis, which can occur following Impella® ventricular assist device (Abiomed®) deployment for cardiogenic shock management. While no randomized controlled trials (RCTs) or formal societal guidelines currently endorse or refute the use of the Penumbra Indigo System in this specific setting, several case series support its technical feasibility and safety.
It is important to note that routine manual aspiration thrombectomy during percutaneous coronary intervention (PCI) is generally not recommended due to a lack of demonstrated clinical benefit and a potential increased risk of stroke. However, continuous mechanical thrombectomy with the CAT™ RX catheter (Penumbra) has shown meaningful clinical benefit, with the CHEETAHi study reporting high technical success and no device-related serious adverse events, including stroke. This case highlights a successful intervention using the Penumbra Indigo System for femoral artery thrombosis, reinforcing its utility in carefully selected clinical scenarios.
Case Presentation
A 45-year-old man with a history of polysubstance abuse (cocaine, tetrahydrocannabinol, alcohol, and recent heroin use) presented with intractable nausea and vomiting. In the emergency department, the patient experienced worsening respiratory distress, necessitating intermittent noninvasive ventilation. Initial vitals revealed a blood pressure of 151/88 mm Hg, heart rate of 59 bpm, oxygen saturation >90%, and a mildly elevated troponin I of 0.07 ng/mL.
Despite improvements in emesis following administration of droperidol and trimethobenzamide, the patient developed chest pain. A repeat ECG demonstrated anterior ST-elevation myocardial infarction with ST elevation in V1-V3 and reciprocal ST depressions in leads II, III, and aVF. Due to anticipated delays in transfer to a PCI-capable facility, tenecteplase was administered as per standard guidelines.
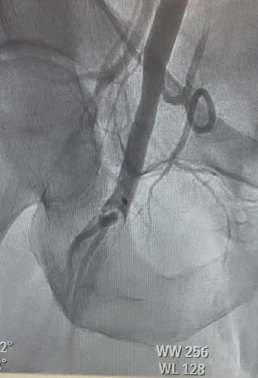
On arrival to our center, the patient continued to report chest pain (5/10). Troponin I had peaked to 31 ng/mL, and serum lactic acid was elevated. Repeat ECG showed resolution of ST elevations, but clinical suspicion remained high for cardiogenic shock. The patient was intubated and mechanically ventilated, and a periprocedural Impella 2.5 left ventricular assist device was inserted for circulatory support. Angiography confirmed left anterior descending (LAD) patency but with significant thrombus burden (Figure 1). Coronary mechanical thrombectomy was performed using the CAT RX catheter. Lesion preparation was carried out with a 4.0 x 12 mm Shockwave balloon at the ostial LAD, followed by deployment of a 4.0 x 18 mm drug-eluting stent extending from the ostial to the proximal LAD. The following day, the patient demonstrated significant clinical improvement, with an ECG showing normalization of ventricular function and ejection fraction. Following removal of the Impella, homeostasis was achieved; however, the right lower extremity appeared dusky with an absence of palpable and Doppler-detectable pulses.
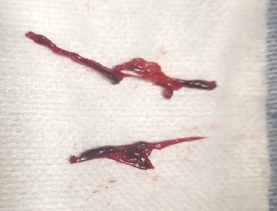
Urgent intervention was undertaken. Left femoral access was obtained, and a 6F sheath was inserted for up-and-over access. A 5F Omni™ Flush catheter (AngioDynamics) was advanced into the right external iliac artery, and angiography revealed thrombus surrounding the previously placed MANTA closure device (Teleflex®). The catheter was exchanged for a 7F, 45-cm Pinnacle sheath (Terumo), and a 7F Lightning Bolt® aspiration catheter (Penumbra) was advanced for clot retrieval. The thrombus “lollipopped” onto the catheter; therefore, the entire aspiration system, including the sheath, was removed. A significant amount of clot was extracted (Figure 2).
Manual pressure was applied to achieve hemostasis at the left femoral access site. Following the procedure, pulses were palpable and +2 bilaterally. The patient tolerated the intervention well and left the catheterization lab in stable condition. This highlighted a very important intervention and the use of the Penumbra coronary CAT RX and peripheral Lightning Bolt System for multiple successful interventions.
Discussion
Aspiration thrombectomy using a Penumbra catheter for clot removal is well-supported in the literature for coronary as well as arterial and venous applications. Multiple reports describe percutaneous aspiration thrombectomy (PAT) as a safe and effective strategy for managing arterial thromboembolic complications in the lower extremities. Guidelines note that PAT can be performed as a standalone intervention or in conjunction with thrombolytic therapy. SIR has endorsed this approach as part of quality improvement initiatives for the treatment of acute limb ischemia (ALI), including embolic occlusions that occur post-endovascular procedures.
Clinical series have demonstrated success rates as high as 95.7% for PAT in infrainguinal thromboembolic occlusions occurring during or after endovascular interventions, with favorable limb salvage outcomes and relatively low complication rates. The Penumbra Indigo System has been used for mechanical aspiration thrombectomy in both coronary and peripheral arterial territories. Case series have shown it to be safe and effective for thrombus removal in the context of PCI and ALI.
Recent studies have demonstrated favorable clinical outcomes with the Penumbra Indigo aspiration system in the management of ALI. For example, a prospective multicenter study involving 119 patients reported a 30-day limb salvage rate of 98.2% and primary patency rate of 89.4% at 1 month according to the authors of the STRIDE trial. A key highlight was the rate of major amputations was exceptionally low and device-related complications were rare.
The INDIAN registry, another prospective observation study of 150 patients, indicated a 92% primary patency at 1 month and a major amputation rate below 1% using the Indigo System. This reinforced the effectiveness and safety profile on the table for Penumbra.
Vacuum-assisted aspiration and rheolytic technology are both FDA-cleared endovascular thrombectomy devices used for management of ALI, the former being the Indigo System. The AngioJet™ system (Boston Scientific) employs a high-pressure saline jet generating a venturi effect to fragment and remove thrombus. Like Indigo, it can be used as standalone therapy or in addition to thrombolytics. The vacuum-assisted aspiration model of Indigo mechanically extracts thrombotic material from the vasculature in comparison.
A review of the evidence from multicenter registries and case series have shown that the AngioJet system achieves substantial or complete revascularization in approximately 70% of cases, with 1-year primary patency rates between 69% and 74%, but overall technical and clinical success rates range from 92% to 93%, and limb salvage rates are very similar in select studies.
The Indigo aspiration device has demonstrated at least comparable, if not superior, outcomes in terms of technical success, limb salvage, and a good safety profile. Although there are no large-scale RCTs head-to-head that exist to definitively compare the different modalities, the 2 devices do not share similar safety profiles and here is where we can choose a horse in the race. However, STRIDE showed a low incidence of major bleeding (4.2%), with none of these events reported as related to the use of the Indigo system.
Additionally, STRIDE reported an extremely low rate of device-related adverse events (0.8%). Risk of hemolysis and kidney injury are minimal, and complications published in the literature (distal embolization, hematomas) are uncommon and manageable.
In contrast, the AngioJet system carries a risk of hemolysis, which can lead to acute renal injury due to the release of free hemoglobin, and this risk is more prevalent with prolonged device activation, which led the manufacturer to recommend time of use under 10 minutes. There are also access site complications and fluid overload concerns.
The choice of thrombectomy system deployed may ultimately depend on operator preference and clinical presentation, as both devices have demonstrated low rates of major amputation and mortality in the current clinical world. Direct comparative trials are warranted. Another platform to mention, the Rotarex™ system (BD), is also supported by multiple studies for ALI and is well established in Europe, but less commonly seen in US-based guidelines. The Pounce™ thrombectomy system (Surmodics) is a newer device with limited published data, primarily small case series and a single-center retrospective study in ALI.
Conclusion
Given the lack of RCT data, we emphasize that such interventions remain highly effective with a good safety profile for ALI using the Penumbra Indigo System, and more data should be obtained for patients undergoing PCI who may also require a mechanical thrombectomy.
Affiliation: The authors are from East Carolina Heart Institute at East Carolina University in Greenville, North Carolina.
This manuscript was sponsored by Penumbra, Inc.
Procedural and operative techniques and considerations are illustrative examples from physician experience. Physicians’ treatment and technique decisions will vary based on their medical judgment. The clinical results presented herein are for informational purposes only and may not be predictive for all patients. Individual results may vary depending on patient-specific attributes and other factors.
Caution: Federal (USA) law restricts these devices for sale by or on the order of a physician. Prior to use, please refer to the Instructions for Use for complete product indications, contraindications, warnings, precautions, potential adverse events, and detailed instructions for use. For the complete Penumbra IFU Summary Statements, please visit www.peninc.info/risk. Please contact your local Penumbra representative for more information.
References
Costopoulos C, Gorog DA, Di Mario C, Kukreja N. Use of thrombectomy devices in primary percutaneous coronary intervention: a systematic review and meta-analysis. Int J Cardiol. 2013;163(3):229-241. doi:10.1016/j.ijcard.2011.11.014
Crimi G, Moramarco L, Mandurino-Mirizzi A, Quaretti P, Ferrario M. The combined use of stent retriever and neuro-aspiration as successful bail-out strategy in embolic myocardial infarction. Catheter Cardiovasc Interv. 2019;94(2):E78-E81. doi: 0.1002/ccd.28167
de Donato G, Pasqui E, Sponza M, et al; INDIAN Trial Collaborators. Safety and efficacy of vacuum assisted thrombo-aspiration in patients with acute lower limb ischaemia: the INDIAN trial. Eur J Vasc Endovasc Surg. 2021;61(5):820-828. doi:10.1016/j.ejvs.2021.01.004
De Luca G, Navarese EP, Suryapranata H. A meta-analytic overview of thrombectomy during primary angioplasty. Int J Cardiol. 2013;166(3):606-612. doi:10.1016/j.ijcard.2011.11.102
Hussain SI, Zaidat OO, Fitzsimmons BM. The Penumbra system for mechanical thrombectomy in endovascular acute ischemic stroke therapy. Neurology. 2012;79(13 Suppl 1):S135-S141. doi:10.1212/WNL.0b013e31826958a8
Kadakia KT, Beckman AL, Ross JS, Krumholz HM. Renewing the call for reforms to medical device safety—the case of Penumbra. JAMA Intern Med. 2022;182(1):59-65. doi:10.1001/jamainternmed.2021.6626
Kumbhani DJ, Bavry AA, Desai MY, Bangalore S, Bhatt DL. Role of aspiration and mechanical thrombectomy in patients with acute myocardial infarction undergoing primary angioplasty: an updated meta-analysis of randomized trials. J Am Coll Cardiol. 2013;62(16):1409-1418. doi:10.1016/j.jacc.2013.04.025
Maldonado TS, Powell A, Wendorff H, et al; STRIDE Study Group. Safety and efficacy of mechanical aspiration thrombectomy for patients with acute lower extremity ischemia. J Vasc Surg. 2024;79(3):584-592.e5. doi:10.1016/j.jvs.2023.10.062
Mathews SJ, Brown CL, Kolski BC, et al. Initial experience with a continuous mechanical aspiration system for thrombus removal before percutaneous coronary intervention. Catheter Cardiovasc Interv. 2022;100(6):950-954. doi:10.1002/ccd.30389
Mathews SJ, Parikh SA, Wu W, et al. Sustained mechanical aspiration thrombectomy for high thrombus burden coronary vessel occlusion: the multicenter CHEETAH study. Circ Cardiovasc Interv. 2023;16(2):e012433. doi:10.1161/CIRCINTERVENTIONS.122.012433
Patel NH, Krishnamurthy VN, Kim S, et al; CIRSE and SIR Standards of Practice Committees. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24(1):3-15. doi:10.1016/j.jvir.2012.09.026
Peng S, Rempakos A, Mastrodemos OC, et al. Use of the Indigo CAT RX aspiration system during percutaneous coronary intervention. Catheter Cardiovasc Interv. 2024;103(5):695-702. doi:10.1002/ccd.30994
Penumbra Pivotal Stroke Trial Investigators. The Penumbra Pivotal Stroke Trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40(8):2761-2768. doi:10.1161/STROKEAHA.108.544957
Schleder S, Diekmann M, Manke C, Heiss P. Percutaneous aspiration thrombectomy for the treatment of arterial thromboembolic occlusions following percutaneous transluminal angioplasty. Cardiovasc Intervent Radiol. 2015;38(1):60-64. doi:10.1007/s00270-014-0857-6
Sirker A, Mamas M, Kwok CS, et al; British Cardiovascular Intervention Society (BCIS). Outcomes from selective use of thrombectomy in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an analysis of the British Cardiovascular Intervention Society/National Institute for Cardiovascular Outcomes Research (BCIS-NICOR) registry, 2006-2013. JACC Cardiovasc Interv. 2016;9(2):126-134. doi:10.1016/j.jcin.2015.10.047
Tamhane UU, Chetcuti S, Hameed I, Grossman PM, Moscucci M, Gurm HS. Safety and efficacy of thrombectomy in primary percutaneous coronary intervention for acute ST elevation MI: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2010;10:10. doi:10.1186/1471-2261-10-10
Wei L, Zhu Y, Liu F, et al. Infrainguinal endovascular recanalization: risk factors for arterial thromboembolic occlusions and efficacy of percutaneous aspiration thrombectomy. J Vasc Interv Radiol. 2016;27(3):322-329. doi:10.1016/j.jvir.2015.11.025
iThe safety and effectiveness of this device for use in the treatment of ST-Elevation Myocardial Infarction (STEMI) has not been established. Complications from the use of this device in this manner could lead to death, permanent impairment, and/or the need for emergency medical intervention.










