VINTAGE: A Future Guidewire-Based Ablation Technique for Deep Targets?
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of EP Lab Digest or HMP Global, their employees, and affiliates.
EP LAB DIGEST. 2025;25(12):6,20.
Bradley P Knight, MD, FACC, FHRS
Dear Readers,
In the November 2025 issue of EP Lab Digest, novel methods to ablate ventricular arrhythmias arising from deep in the myocardium were reviewed.1 These methods include needle-based irrigated ablation catheters, external radiation therapy, ultra-low cryoablation, and coronary venous alcohol injection. The focus was a new pulsed field ablation (PFA) catheter capable of creating deep myocardial lesions and the results of the recently published Ventricular Catheter Ablation Study (VCAS) that described deep transmural ventricular PFA lesions in humans using a novel 8.5 French, force-sensing, high-voltage PFA catheter (FieldForce PFA System; Field Medical).2
At Electrophysiology (EP) in the West 2025 on October 24, 2025, Neal Bhatia, MD, from Emory University, presented a novel method for ablating deep myocardial targets using intramyocardial guidewires. He described an impressive and intriguing method of steering guidewires within the muscle of the left ventricle (LV) through a sheath anchored at the right ventricular (RV) septum to deliver a deep intramural ablation electrode anywhere within the LV, a procedure known as VINTAGE (Ventricular Intramyocardial Navigation for Tachycardia Ablation Guided by Electrograms). His group partnered with Robert Lederman, MD, and colleagues at the National Institutes of Health and published their findings in JACC: Clinical Electrophysiology in October 2025.3 In their retrospective review, 13 patients with an average of 2 prior failed ablations for either ventricular tachycardia (VT; 5 patients) or premature ventricular contractions (PVCs; 8 patients) underwent VINTAGE. Ablation targets included the LV summit (n = 3), basal-lateral wall (n = 4), septum (n = 3), inferior wall (n = 1), and papillary muscles (n = 2). Technical success was achieved in 100% of cases, and at a median follow-up of 150 days, clinical success was 85%.
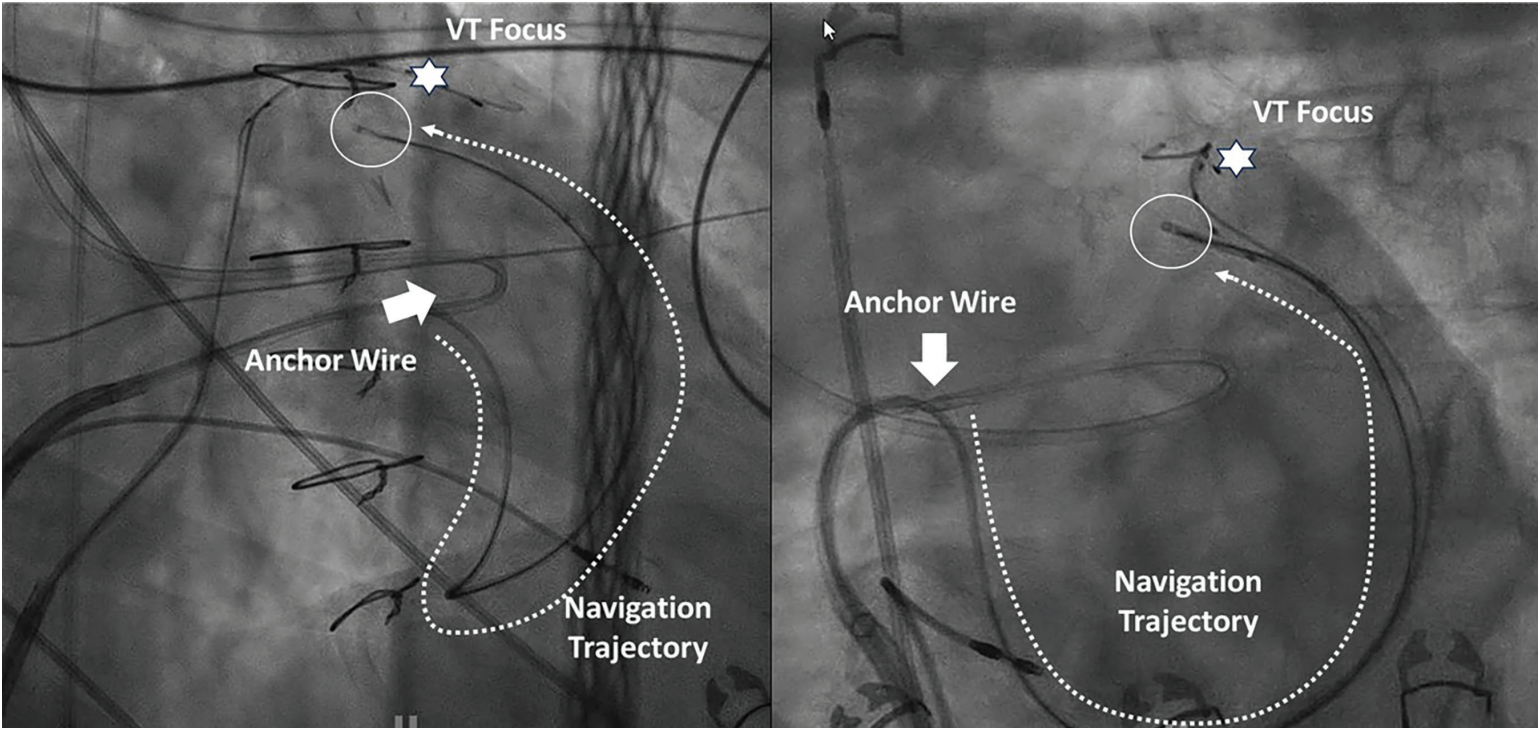
The VINTAGE ablation procedure is complex and involved. It requires placement of a deflectable sheath against the RV septum, puncturing into the LV, and using a snare from the femoral artery to create a rail. This allows the delivery sheath to be firmly applied to the septum. A stiff coronary guidewire, typically used to open chronic total occlusions (CTO), is then placed through a microcatheter and steered throughout the myocardium. Notably, electrophysiologists can direct the guidewire in nearly any direction within the muscle to get close to the target. Rotations from the femoral vein are able to be transmitted to the tip of the wire, with minimal resistance when advancing the tip within the muscle. The guidewire is navigated using fluoroscopy, echocardiography, and a novel electrogram-based technique known as Electrocardiographic Radial Depth Navigation (EDEN). In a prior study of EDEN, Bruce et al demonstrated that characteristics of the electrograms recorded from the tip of the wire can indicate whether the tip is endocardial, midmyocardial, or epicardial: “EDEN signatures distinguished 5 depth zones throughout left and right ventricular free walls and interventricular septum. Relative ST-segment elevation magnitude best discriminated position and was maximum (40.1 ± 6.5 mV) in the midmyocardium. Subendocardial positions exhibited dominant Q waves with lower-amplitude ST segments (16.8 ± 5.8 mV), whereas subepicardial positions exhibited dominant R waves with lower-amplitude ST segments.”4 Once the target is reached, the CTO wire is exchanged for a larger wire used to deliver radiofrequency (RF) ablation lesions. (Figure, Video)
Many failed ablation cases for VT and PVCs occur because the target is too deep to be reached with standard RF ablation lesions. The VINTAGE approach represents another innovative concept that has demonstrated potential in the EP lab. Although the term “vintage” commonly describes something from a past era, in the case of this new guidewire-based navigation and ablation technique, the acronym VINTAGE may instead signify a step toward the future.
Video
Illustration of intramyocardial navigation of counterclockwise navigation real time from septum to left ventricular summit region. (Courtesy of Neal Bhatia, MD.)
References
- Knight BP. Pulsed field ablation for ventricular arrhythmias—going deep. EP Lab Digest. 2025;25(11):6.
- Reddy VY, Koruth, JS, Peichl P, et al. High-voltage focal pulsed field ablation to treat scar-related ventricular tachycardia: the first-in-human VCAS trial. Circulation. Oct 10. Online ahead of print. doi:10.1161/CIRCULATIONAHA.125.077025
- Bhatia NK, Halaby RN, Bruce CG, et al. Ventricular intramyocardial navigation for tachycardia ablation guided by electrograms (VINTAGE): initial human experience. JACC Clin Electrophysiol. 2025;11(10):2137-2150. doi:10.1016/j.jacep.2025.06.003
- Bruce CG, Yildirim DK, Kolandaivelu A, et al. EDEN (electrocardiographic radial depth navigation): a novel approach to navigate inside heart muscle. JACC Clin Electrophysiol. 2023;9(8 Pt 3):1741-1754. doi:10.1016/j.jacep.2023.04.016
Disclosures: Dr Knight has served as a paid consultant to Medtronic and was an investigator in the PULSED AF trial. He has served as a consultant, speaker, investigator, and/or has received EP fellowship grant support from Abbott, AltaThera, AtriCure, Baylis Medical, Biosense Webster, Biotronik, Boston Scientific, CVRx, Philips, and Sanofi; he has no equity or ownership in any of these companies. Dr Knight reports payment or honoraria from Convatec for a lecture.











