Pulsed Field Ablation for Ventricular Arrhythmias—Going Deep
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of EP Lab Digest or HMP Global, their employees, and affiliates.
EP LAB DIGEST. 2025;25(11):6.
Bradley P Knight, MD, FACC, FHRS
Dear Readers,
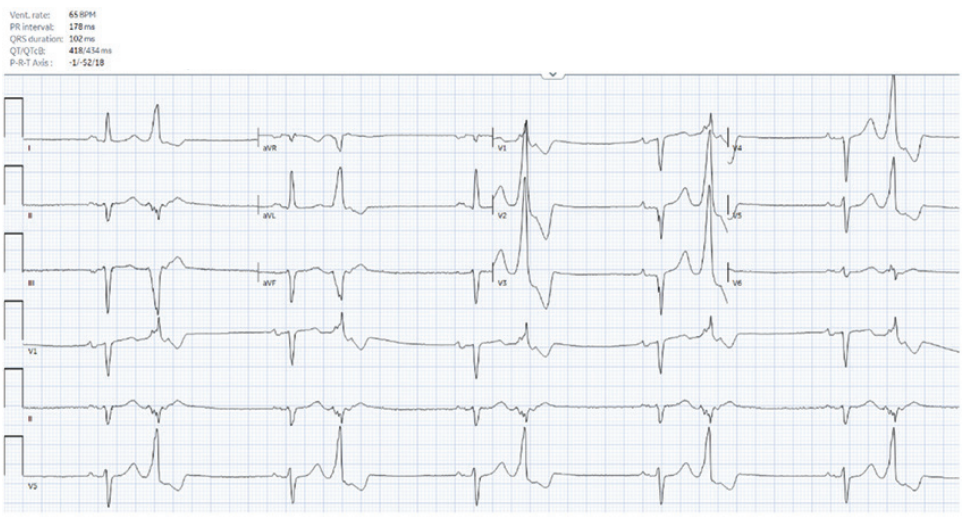
A 54-year-old man with coronary artery disease status post stenting of the right coronary artery and systemic sclerosis with subendocardial fibrosis of the mid and basal inferoseptum and inferior wall on cardiac magnetic resonance imaging was referred for frequent, highly symptomatic, unifocal, premature ventricular contractions (PVCs) with a right bundle superior axis morphology. He was in ventricular bigeminy when seen in clinic (Figure 1) and had a PVC burden of 27% on a recent Holter. He underwent an attempted ablation procedure a few months earlier, during which the PVCs were mapped to the posterior papillary muscle and inferior left ventricular (LV) base. Activation times were reported to range from 0 to -20 milliseconds pre-QS; however, ablation was unsuccessful. What factors contributed to the unsuccessful ablation, and what considerations should be made for the redo procedure?
One possibility that the initial procedure was unsuccessful is that the PVCs were coming from the papillary muscle, where catheter stability using a standard radiofrequency (RF) electrode tip catheter can be challenging. A second possibility is that additional mapping was required to more accurately locate the site of origin. A third possibility is that the PVCs originated deep to the endocardium, beyond the effective reach of RF energy. Could pulse field ablation (PFA) offer a solution?
The patient underwent a repeat procedure using a 9-mm lattice-tip, dual-energy RF/PF ablation catheter (Affera Mapping System, Medtronic) with a transseptal approach guided by intracardiac echocardiography. This approach enabled ultra-high-density mapping of the heads of the posterior papillary muscle and surrounding area (Figure 2 and video). At a site near the mitral annulus at approximately the 6:30 position, where an atrial electrogram was recorded during sinus rhythm, ventricular activation of the PVC occurred approximately 15 milliseconds pre-QRS. A single RF lesion eliminated the PVCs. A second RF lesion and a single PFA lesion were delivered. There was no recurrence of PVCs during the overnight period.
Successful ablation during the repeat procedure may have been due to targeting a different site or possibly creating a deeper lesion. However, the current 9-mm lattice-tip system creates lesions that are limited to 5 or 6 mm in depth. Ablation was successful in this patient; however, what about patients with ventricular tachycardia (VT) circuits that are a centimeter away from the endocardium? A tool that can deliver deeper lesions would be useful.
In October 2025, Reddy et al1 published the Ventricular Catheter Ablation Study (VCAS), the first-in-human investigation of catheter ablation for scar-related VT using a novel 8.5 French, force-sensing, high-voltage PFA catheter (FieldForce PFA System, Field Medical). Preclinical studies have demonstrated that this catheter can create deep myocardial ablation lesions. In this study, 5 applications of PFA lesions were delivered at each site. Repetitive epicardial mapping was performed before and after endocardial PFA in a subset of 10 patients and demonstrated transmural lesion formation. There was a 98% reduction in the burden of ventricular arrhythmias post ablation and the few complications that occurred were not clearly specific to the energy source. The ability to create transmural LV lesions with PFA from the endocardium represents a breakthrough.
There are many patients with difficult-to-treat PVCs and VT due to LV scar related to circuits that are far from the endocardial surface and are challenging to ablate with conventional RF technology. There are limits to RF ablation. Alternatives to epicardial ablation currently under development include needle-based irrigated RF catheters, external radiation therapy, ultra-low-temperature cryoablation, and coronary venous alcohol injection. VCAS demonstrated that novel PFA systems can be used to create deep transmural lesions from the LV endocardium. Although avoiding excessive ablation with these PFA tools will be important, they may offer a new option for some patients with VT that is refractory to conventional endocardial RF ablation.
Disclosures: Dr Knight has served as a paid consultant to Medtronic and was an investigator in the PULSED AF trial. He has served as a consultant, speaker, investigator, and/or has received EP fellowship grant support from Abbott, AltaThera, AtriCure, Baylis Medical, Biosense Webster, Biotronik, Boston Scientific, CVRx, Philips, and Sanofi; he has no equity or ownership in any of these companies. Dr Knight reports payment or honoraria from Convatec for a lecture.











