Plug and Play: Accelerating Same-Day Discharge After EP Procedures
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of EP Lab Digest or HMP Global, their employees, and affiliates.
EP LAB DIGEST. 2025;25(11):1,18-20.
Mohamed Elshazly, MD, MBEE
Orlando Health Heart and Vascular Institute, Longwood, Florida
Electrophysiology (EP) programs are judged first by procedural success and safety. Yet, in a busy lab, the day-to-day realities of hospital-acquired infection (HAI), patient experience, and EP lab efficiency and throughput carry equal weight. HAIs reliably lengthen hospital stays and readmission, and are associated with inflating costs.1,2 The needs of safely shorting bed rest and observation time, as well as same-day discharge (SDD), are imperative for any successful EP laboratory and endorsed by the American College of Cardiology.3
The use of traditional manual compression for venous vascular closure often means prolonged supine time and routine indwelling urinary catheters; both are linked to catheter-associated urinary tract infection (UTI) risk during atrial fibrillation (AF) ablation and, by extension, avoidable infection-related delays.4 Device-based venous closure, by contrast, is explicitly designed to enable earlier hemostasis and ambulation after multi-site femoral venous access, an approach supported by randomized EP data.5,6
While freedom from arrhythmia headlines success, the patient experience at discharge is frequently dictated by their access site complications, time to walk, and readiness to go home. Vascular access complications remain the most common adverse events after AF ablation and are associated with reduced comfort, lower quality of life, longer stays, higher costs, and increased rates of readmission.7 The MYNX CONTROL VENOUS Vascular Closure Device (MYNX CONTROL VENOUS VCD, Cordis) directly addresses this with its novel, user-friendly delivery of polyethylene glycol (PEG) sealant to the venotomy, improving hemostasis, ambulation, and discharge eligibility times compared to manual compression.6 These improvements corroborate findings from meta-analyses that SDD after AF ablation is feasible, safe in appropriately selected patients, and cost-effective.8,9 These data reinforce that reliable vascular closure is not a technical afterthought but rather a critical determinant of infection risk, patient satisfaction, and timely discharge in the modern EP lab.
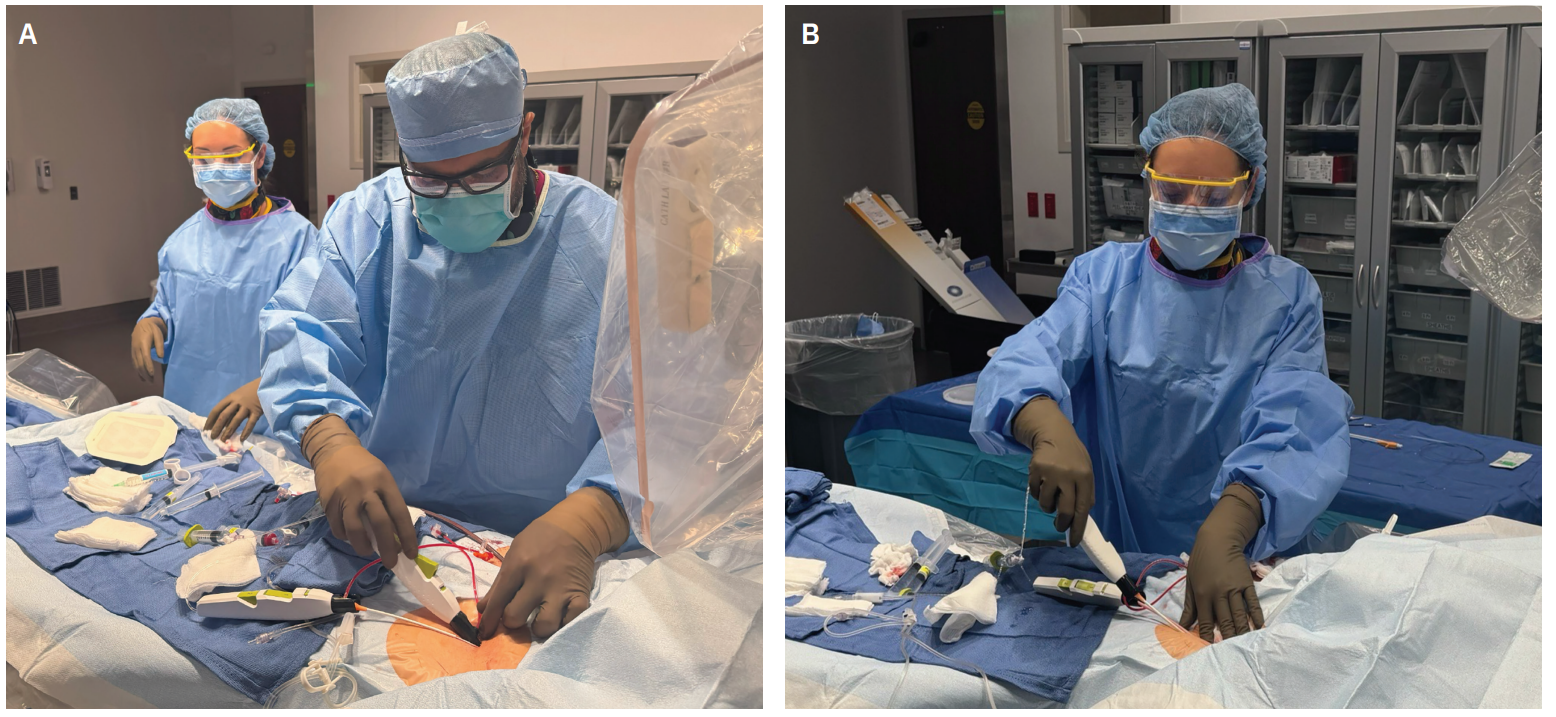
Case Presentation
The patient is an 81-year-old female with prior history of paroxysmal AF, treated with cryoballoon pulmonary vein isolation (PVI) in 2021, as well as hypertension and type 2 diabetes mellitus, who experienced multiple hospital readmissions for atypical atrial flutter (AFL) with rapid ventricular response. She did not tolerate multiple antiarrhythmic medications due to side effects and decided to pursue AFL ablation and redo PVI. While on anticoagulation with a direct oral anticoagulant (DOAC), she had 2 episodes of upper gastrointestinal bleeding related to peptic ulcer disease, and one of these episodes required hospital admission and blood transfusion. Her CHA2DS2-VASc score was 5 and HAS-BLED score was 4. Through a shared decision-making process, we elected to pursue concomitant ablation and left atrial appendage occlusion (LAAO) with a Watchman device (Boston Scientific) in the same setting.
During our discussion in clinic, the patient and their family expressed a preference for a brief period of bed rest after the procedure and indicated interest in SDD. We discussed using the MYNX CONTROL VENOUS VCD for hemostasis following ablation, noting that our prior experience with this device has allowed for SDD in a majority of our patients with early ambulation 2 hours postoperatively. It has been our experience that the MYNX CONTROL VENOUS VCD is associated with a lower risk of hematoma, manual pressure time, closure failure, and late bleeding. For this case, radiofrequency (RF) ablation was utilized, consisting of the QDot Ablation Catheter (Johnson & Johnson MedTech) through an 8.5 French (F) Vizigo Sheath (Johnson & Johnson MedTech). This was exchanged for a 14F delivery sheath (Watchman Double Curve) for the second part of the procedure.
Vascular Access
The patient presented with atypical AFL. General anesthesia was administered under physician/nurse guidance. The patient was prepared and draped in standard fashion. Cephalexin, 2g intravenously (IV), was administered for antibiotic prophylaxis. Using the modified Seldinger technique under ultrasound guidance, an 11F, 25-cm Pinnacle Introducer Sheath (Terumo Interventional Systems) was inserted into the left common femoral vein, a short 7F Pinnacle Introducer Sheath (Terumo) was placed into the left common femoral vein, and an 8F short Pinnacle Introducer Sheath (Terumo) was placed through the right common femoral vein.
Procedural Considerations
We proceeded with our regular workflow for concomitant ablation and LAAO with the Watchman device. A 10F 4D Intracardiac Echocardiography (ICE) Nuvision Ultrasound Catheter (Johnson & Johnson MedTech) was advanced through the 11F left femoral vein sheath into the right atrium, and a DecaNav Decapolar Catheter (Johnson & Johnson MedTech) was advanced through the 7F sheath and placed in the coronary sinus. The AFL had a cycle length of 260 ms with variable atrioventricular conduction and concentric activation. Entrainment maneuvers showed the AFL was most likely left sided and roof dependent. IV heparin was administered for a target activated clotting time (ACT) of 350-400 seconds for the duration of the procedure.
The 8F right femoral vein sheath was exchanged for the 8.5F Vizigo sheath. Transseptal access (low and mid septum) was performed using ICE and fluoroscopic guidance via the VersaCross RF Wire (Boston Scientific) and Vizigo sheath. Using the same septostomy, the ICE catheter was advanced into the LA to guide the concomitant ablation and Watchman implant. An Octaray Mapping Catheter (Johnson & Johnson MedTech) was used to create a 3-dimensional anatomical shell using the Carto 3 System Version 8 (Johnson & Johnson MedTech) of the LA and to delineate the anatomy of all PVs. It was also used to create an activation map, which confirmed that the AFL was dependent on the posterior wall (PW) through a narrow channel between the prior PVI lines of the left and right veins. The Octaray catheter was removed, and the QDot Ablation Catheter was advanced to the LA and used to ablate the roof and floor lines isolating the PW. During roof ablation, the AFL terminated. All PVs were confirmed to have exit and entrance block. After the ablation, the ablation catheter was removed, and the Octaray catheter was used to remap the LA, showing sharply demarked scar along the ablation lines with confirmed isolation (entrance and exit block) of all 4 PVs and the PW. Thereafter, the AFL was not inducible with pacing maneuvers and isoproterenol.
We then proceeded with the Watchman implant. The Vizigo sheath was exchanged over the VersaCross RF Wire with a 14F double curve Watchman delivery system. Angiography was performed to confirm LAA anatomy seen on ICE, consistent with a windsock morphology. Based on ICE and fluoroscopy sizing, a 27-mm Watchman FLX Pro Device was placed through the Watchman delivery system and deployed in the LAA. The device was then released after proper positioning, anchoring, sealing, and compression criteria were confirmed.
Vascular Closure With MYNX CONTROL VENOUS VCD
Protamine was administered to partially reverse the ACT. The 11F, long 25-cm sheath in the left groin and the Watchman 14F delivery sheath were exchanged over a long wire for 2 short 11F sheaths to prepare for closure with the MYNX CONTROL VENOUS VCD. Light manual pressure was held over the 11F sheath in the right groin for 2 minutes, and there was no bleeding around the sheath after downsizing. The MYNX CONTROL VENOUS VCD was then prepped and inserted into the right 11F short sheath. The sheath catch sidearm was wrapped around the side port of the short sheath. The device’s 6-mm semi-compliant balloon was fully inflated with saline with the locking syringe, and no resistance was felt. Full inflation was also confirmed with visualization of the “white-black-white” indicator on the proximal end of the MYNX CONTROL VENOUS VCD. The device and sheath were gently withdrawn with steady traction using the right hand, drawing the inflated intraluminal balloon toward the venotomy site. Proper technique involves holding the device in the right hand with its buttons facing upward, while the left hand is placed at the groin to provide light, stabilizing downward pressure as the device and sheath are retracted. Once the sheath has been withdrawn and the balloon is felt to be at the venotomy site with light countertraction, the tension indicator comes into alignment and button 1 is pressed fully to release the PEG hydrogel at the venotomy site.
At this point, a 2-minute timer was started, providing time for the water-soluble sealant to expand 3 to 4 times its dehydrated size within the tissue tract and seal the venotomy. The other 2 MYNX CONTROL VENOUS VCDs were deployed in the left groin through 7F and 11F short sheaths using the same technique. Saline was used to irrigate the venotomy sites by expelling 2 to 3 ml over the venotomy sites, further facilitating activation and expansion of the sealant.
Due to the MYNX CONTROL VENOUS VCD’s consistent ease of use and reproducible safety and efficacy profile, the technologists in our facility support the removal of the device following deployment. Following the 2-minute dwell time, the technologist deflated the balloons and applied light manual pressure on the venotomy sites for stabilization. The device was held in the right hand while depressing button 2 to retract the balloon prior to completely removing the device and sheath from the tissue tract. The technologist held manual pressure for another 1 to 2 minutes until hemostasis was achieved. Lidocaine 1% was then administered at both femoral access sites for local anesthesia and pain control. The entire closure process took approximately 5 to 6 minutes, with hemostasis achieved and no immediate or late rebleeding observed during recovery. A DOAC was administered once the patient was sufficiently awake to take oral medications. After 2 hours of bed rest, the patient was able to ambulate without any difficulties, pain, or hematoma in their femoral access sites. The patient was discharged the same day and experienced an excellent postoperative course.
The patient did not have recurrence of AF or AFL, and 6-week postoperative transesophageal echocardiogram confirmed appropriate placement of the Watchman device without any peri-device leaks or thrombus. Anticoagulation was stopped after 3 months, and the patient was transitioned to a dual antiplatelet regimen for 3 months followed by an aspirin-only regimen.
Discussion
In contemporary EP workflows, emerging data suggest ablation9-12 and LAAO13,14 procedures are safe and feasible for SDD. However, the primary barrier to SDD remains achieving reliable, rapid hemostasis without compromising safety, particularly when multiple large-bore femoral venous access sites are required for ablation and LAAO procedures. Furthermore, with a growing desire to combine ablation and LAAO procedures to further reduce costs and staged procedures,15 optimal closure is necessary for safe and effective postoperative care. The randomized ReliaSeal Trial directly addressed these needs. Compared with manual compression, the MYNX CONTROL VENOUS VCD shortened mean time to hemostasis (2.1 vs 11.4 minutes), time to ambulation (2.6 vs 5.1 hours), and time to discharge eligibility (3.1 vs 5.5 hours), while achieving 100% device and procedural success and lower access site complications at 30 days (major 0.0% vs 0.8%; minor 0.0% vs 5.0%), which were adjudicated by a clinical events committee.6 In practical terms, these gains compared to manual compression translate into less staff time spent holding pressure, fewer repetitive bedside checks for rebleeding during prolonged bed rest, and reduced concern for late bleeding. These workflow benefits were evident in our case, with early ambulation at ~2 hours, no rebleeding, and SDD. Collectively, our results reflect a shift toward expedited recovery pathways that improve workflow efficiency and patient care.
Disclosure: Dr Elshazly has completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. For the present manuscript, he reports consulting fees from Cordis and honoraria from Johnson & Johnson MedTech. In the past 36 months, he reports consulting fees from Biotronik, honoraria from the Western Atrial Fibrillation Symposium and North Florida Cardiovascular Symposium, and support for attending meetings and/or travel from Abbott, Medtronic, Biotronik, and Cordis.
The transcripts have been edited for clarity and length.
This content was published with support from Cordis.
References
- Rahmqvist M, Samuelsson A, Bastami S, Rutberg, H. Direct health care costs and length of hospital stay related to health care-acquired infections in adult patients based on point prevalence measurements. Am J Infect Control. 2016;44(5):500-506. doi:10.1016/j.ajic.2016.01.035
- Bezerra IL, Nassar Jr AP, Mendonça Dos Santos T, et al; IMPACTO MR Investigators. Patient-level cost analysis of intensive care unit-acquired infections: a prospective cohort study. J Hosp Infect. 2025;159:106-114. doi:10.1016/j.jhin.2024.07.002
- Rao SV, Vidovich MI, Gilchrist IC, et al. 2021 ACC Expert Consensus Decision Pathway on Same-Day Discharge After Percutaneous Coronary Intervention: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6)811-825. doi:10.1016/j.jacc.2020.11.013
- Lewandowski DE, Pierce D, Barnett A, et al. Impact of antibiotic prophylaxis on catheter-associated urinary tract infections during atrial fibrillation ablation. J Interv Card Electrophysiol. 2018;51(2):183-187. doi:10.1007/s10840-018-0325-3
- Natale A, Mohanty S, Liu PY, et al; AMBULATE Trial Investigators. Venous vascular closure system versus manual compression following multiple access electrophysiology procedures: the AMBULATE trial. JACC Clin Electrophysiol. 2020;6(1):111-124. doi:10.1016/j.jacep.2019.08.013
- Summers J, Swarup V, Parker I, et al; other members of the ReliaSeal Study Group. Safety and efficacy of a novel sealant-based vascular closure device following electrophysiology procedures: ReliaSeal Trial. J Cardiovasc Electrophysiol. 2025;36(5):1022-1031. doi:10.1111/jce.16623
- Mohammed M, Ramirez R, Steinhaus DA, et al. Comparative outcomes of vascular access closure methods following atrial fibrillation/flutter catheter ablation: insights from VAscular Closure for Cardiac Ablation Registry. J Interv Card Electrophysiol. 2021;64(2)301-310. doi:10.1007/s10840-021-00981-5
- Jafry AH, Akhtar KH, Khan JA, et al. Safety and feasibility of same-day discharge for catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2022;65(3):803-811. doi:10.1007/s10840-022-01145-9
- Creta A, Ventrella N, Providência R, et al. Same-day discharge following catheter ablation of atrial fibrillation: a safe and cost-effective approach. J Cardiovasc Electrophysiol. 2020;31(12):3097-3103. doi:10.1111/jce.14789
- Theodoreson MD, Chohan BC, McAloon CJ, et al. Same-day cardiac catheter ablation is safe and cost-effective: experience from a UK tertiary center. Heart Rhythm. 2015;12(8):1756-1761. doi:10.1016/j.hrthm.2015.05.006
- Freedman BL, Yang S, Shim D, et al. Feasibility and safety of same-day discharge and shortened bedrest after atrial fibrillation ablation. J Interv Card Electrophysiol. 2022;65(1)209-217. doi:10.1007/s10840-022-01255-4
- Shanker AJ, Jones SO, Blankenship JC, et al. HRS/ACC scientific statement: guiding principles on same-day discharge for intracardiac catheter ablation procedures. Heart Rhythm. 2025;22(6)e1-e12. doi:10.1016/j.hrthm.2025.02.029
- Tan BEX, Boppana LKTB, Abdullah AS, et al. Safety and feasibility of same-day discharge after left atrial appendage closure with the WATCHMAN device. Circ Cardiovasc Interv. 2021;14(1):e009669. doi:10.1161/CIRCINTERVENTIONS.120.009669
- Marmagkiolis K, Ates I, Kose G, Iliescu C, Cilingiroglu M. Effectiveness and safety of same day discharge after left atrial appendage closure under moderate conscious sedation. Catheter Cardiovasc Interv. 2021;97(5):912-916. doi:10.1002/ccd.29376
- Beney J, Galea R, Siontis G, et al. Feasibility study on atrial fibrillation ablation with pulsed field ablation and concomitant occlusion of the left atrial appendage. Europace. 2024;26(7):euae176. doi:10.1093/europace/euae176











