Streamlining PVC Ablation: Practical Strategies for Modern Electrophysiology Labs
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of EP Lab Digest or HMP Global, their employees, and affiliates.
EP LAB DIGEST. 2025;25(12):13-15.
Dinesh Sharma, MD1; Rahul Bhardwaj, MD2; and Jalaj Garg, MD2
1NCH Healthcare System, Naples, Florida; 2Loma Linda University Health, Apple Valley, California
Premature ventricular complex (PVC) ablation is an increasingly common procedure for symptomatic patients with a high PVC burden or PVC-induced cardiomyopathy refractory to medical therapy. This article describes a streamlined workflow implemented in the electrophysiology (EP) labs to improve procedural efficiency, enhance mapping accuracy, and minimize fluoroscopy.
The current guidelines recommend PVC ablation as a first-line therapy for patients with symptomatic PVCs or depressed ejection fraction.1 Improvements in catheter design and mapping strategies, as well as increased operator experience, have made PVC ablation a safe and effective procedure.2 However, procedural success significantly depends on accurately identifying the PVC origin. Conventional radiofrequency (RF) ablation demonstrates lower success rates for epicardial origin and papillary muscle PVCs.3 Preprocedural electrocardiogram (ECG)-based identification of the PVC origin is essential to help patients understand potential risks and anticipated procedural outcomes.
As referring physicians become more aware of PVC ablation, procedural volumes are expected to rise. Thus, establishing an efficient workflow that makes optimal use of tools such as the electroanatomical mapping system is important.
Preprocedural Strategy
Accurate ECG-based localization is critical for informed and shared decision-making. Extensive literature supports ECG localization methods, particularly in identifying challenging PVC origins.4 Left ventricular summit (LVS) PVCs with epicardial origin typically have lower ablation success rates. Similarly, parahisian PVCs carry higher risks for conduction system damage and often yield limited ablation success due to constrained energy delivery.
Evaluating the pre-ablation PVC burden is crucial for objective measurement of procedural success and appropriate patient selection. Generally, patients with a PVC burden below 10% may be managed conservatively unless significantly symptomatic or presenting unclear risk profiles. Procedures are typically more efficient with higher PVC burdens. In certain cases, such as patients with mitral valve prolapse presenting complex PVCs or structural heart disease with complex PVCs despite a low burden, it may be reasonable to perform an EP study for risk stratification and potential ablation.1
Pre-ablation evaluations must include a 12-lead ECG, Holter monitoring, and echocardiogram. Cardiac magnetic resonance imaging is frequently utilized when scar-related PVCs are suspected.
The patients are recommended to hold beta-blockers, calcium channel blockers, or antiarrhythmic medications for at least 3 half-lives to maximize spontaneous PVC occurrence. The sedation is generally minimized to preserve spontaneous PVC occurrence. A small propofol bolus can be administered during femoral venous or arterial access following local infiltration with 10 cc lidocaine. If in the holding area the burden of PVC is low, then isoproterenol is usually started even before the patient enters the EP lab. Intravenous (IV) agents like isoproterenol, phenylephrine, calcium, or epinephrine are judiciously employed to stimulate PVCs if they become suppressed. IV caffeine may also be used when PVCs have clear association with caffeine.
Mapping
Outflow Tract PVCs
In typical right ventricular outflow tract (RVOT) or left ventricular outflow tract (LVOT) PVC cases, the procedure is initiated with the following venous and arterial access points:
- One venous access for intracardiac echocardiography (ICE)
- One venous access for the multipolar mapping catheter (typically a DecaNav), subsequently replaced by the ablation catheter
- An 8 French arterial access for mapping the aortic root and left ventricular inflow below the cusp
Initial ECG analysis guides the expected PVC origin; however, one should also consider anatomical variations. Rapid initial mapping of the RVOT, LVOT, and coronary sinus based on ECG clues efficiently confirms the origin. The procedure begins with mapping of the RVOT using the DecaNav catheter (Johnson & Johnson MedTech). ICE imaging is used initially to contour the aortic cusp, aiding anatomical orientation. ICE with CartoSound (Johnson & Johnson MedTech) constructs a 3-dimensional (3D) shell of the RVOT and adjacent structures, guiding catheter navigation and avoiding critical areas like the left main coronary artery. The DecaNav catheter efficiently captures multiple mapping points at the RVOT anterior, posterior, septal, and lateral positions, providing an initial assessment of PVC origin.
Subsequently, the mapping catheter is inserted in the great cardiac vein to evaluate potential LVS involvement. Earlier activation in the interventricular vein compared to RVOT typically indicates LVOT PVCs. Mapping then proceeds to the aortic root at the right coronary cusp (RCC), left coronary cusp (LCC), and RCC/LCC commissure, followed by regions below the cusp and the aortomitral continuity.
The DecaNav catheter easily prolapses through the aortic valve in a pigtail configuration. To minimize risks, it is recommended to form the pigtail by flexing and clocking the catheter in the descending aorta, avoiding manipulation in the aortic root or ascending aorta to decrease the risk of embolic cerebrovascular accident. Using a long sheath in elderly and patients with peripheral vascular disease helps overcome aortic tortuosity, enhancing torque transmission. Fine mapping of the earliest activation area involves manual annotation of points, aiming for the earliest bipolar electrogram and QS complexes on unipolar signals. Points should be repeatedly verified before ablation to rule out catheter bump or artifact. ICE is frequently used to ensure ablation lesions remain ≥2 cm from the coronary artery.
3D Mapping Display
Mapping is employed using a 3-column display (Figure):
- Column 1: Activation map with local electrogram review
- Column 2: PVC template with morphology match (ideally >97%)
- Column 3: Anatomical map and annotation verification
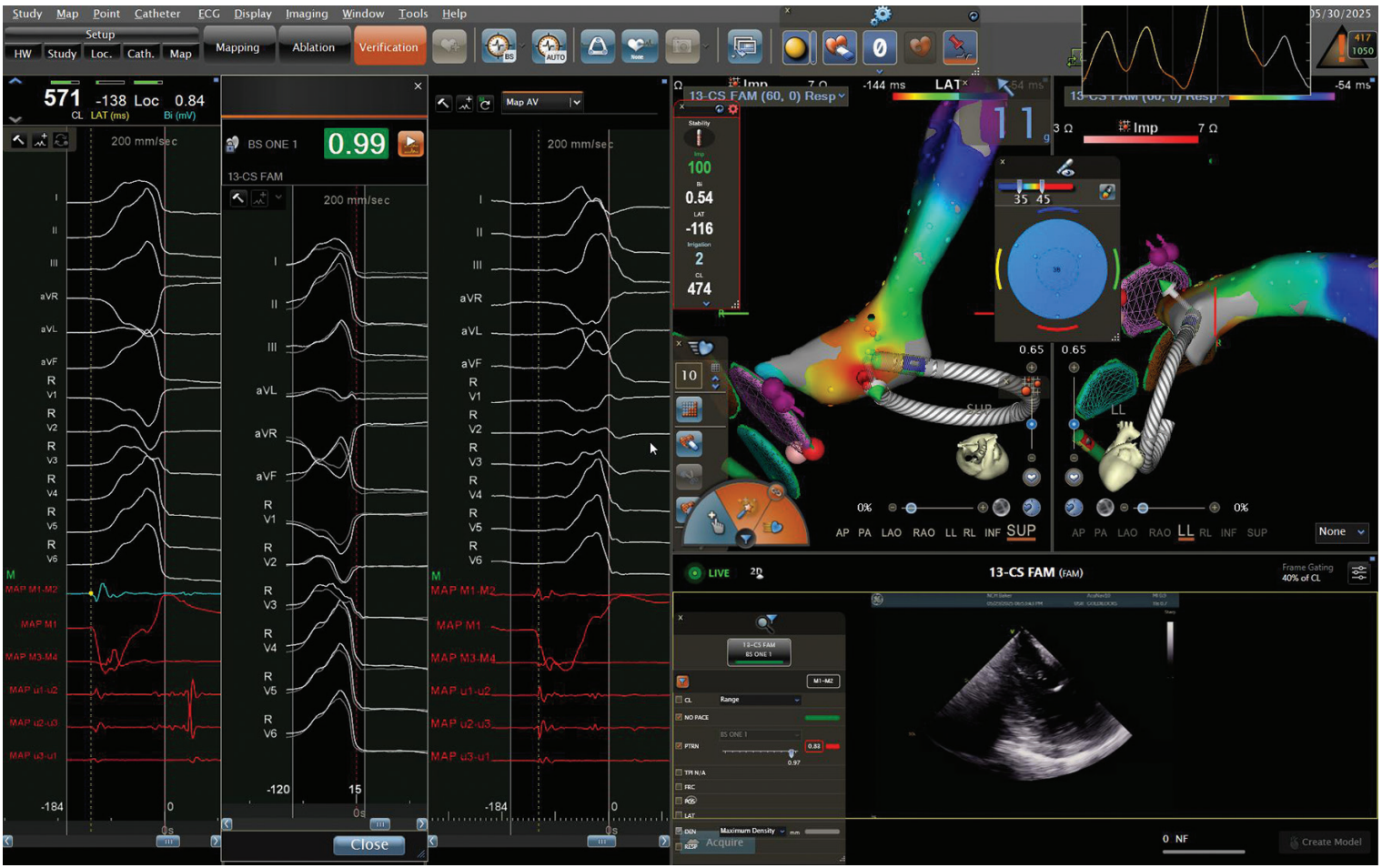
Manual annotation review is strongly encouraged, particularly for fractionated electrograms or poor pace maps, which may indicate deeper or contralateral chamber origins requiring additional mapping (Video 1). Optimal ablation sites feature QS complexes on unipolar signals, earliest activation >20 ms pre-QRS, high-quality pace-map matches, and no coronary artery proximity as confirmed by ICE. If PVC suppression exceeds 5 seconds post ablation, remapping and lesion delivery on the contralateral wall should be considered.
Papillary Muscle and Perimitral PVCs
Papillary Muscle PVCs
Papillary muscle PVCs are common and typically present with a distinct right bundle branch block (RBBB) pattern in lead V1. Anterolateral papillary muscle PVCs usually demonstrate a rightward inferior axis, while posteromedial papillary muscle PVCs typically have a superior and leftward axis. Accurate electrocardiographic identification of PVC origin significantly improves procedural efficiency. Patients, particularly those with underlying mitral valve disease, may also exhibit ventricular arrhythmias originating from the LV basal inferior wall, adjacent to the papillary muscles.
ICE plays a pivotal role in successful mapping and ablation by clearly visualizing papillary muscle anatomy. An ICE catheter is placed in the RV and the CartoSound system is used to create detailed 3D contours of the papillary muscles. Multipolar mapping catheters such as the DecaNav facilitate rapid, comprehensive mapping by collecting multiple points from each position.
The typical mapping display configuration remains consistent, with the system automatically acquiring PVC activation points using a preset morphology-matching threshold (ideally >97%). Frequent ectopy from the papillary muscles, stimulated by catheter contact, helps confirm proximity to the PVC origin. A high pacing-match percentage indicates closeness to the arrhythmogenic focus. Due to inherent instability from contractile tissue, maintaining stable catheter-tissue contact during ablation is challenging. Lower contact forces (<10 grams) are common and compensated by increasing RF power to 50W. Extended duration lesions (>60 seconds) enhance lesion formation despite modest contact.
In cases of suboptimal stability or recurrent arrhythmia, adjunctive cryoablation using an 8-mm cryocatheter (Freezor Cardiac Cryoablation Catheter, Medtronic) may be employed, with direct visualization in the 3D mapping system5 (Video 2). ICE is essential for verifying accurate catheter positioning. For papillary muscle PVCs or ventricular tachycardias, combined RF and cryoablation may reduce recurrence risk. “Insurance lesions” beyond initial ablation success are often beneficial due to the typically lower procedural success rates attributed to instability.
Pulsed field ablation has potential as a future method for papillary muscle arrhythmias, but currently remains experimental.
Perimitral or Basal PVCs
Perimitral and basal PVCs are also distinguishable via 12-lead ECG, commonly exhibiting an RBBB pattern but differing axis compared to papillary muscle PVCs. Papillary muscle PVCs display a distinct rR’ morphology, unlike perimitral or basal PVCs. Anterior mitral annular PVCs typically show lower amplitude in inferior leads compared to outflow PVCs. Inferior basal PVCs could exhibit a V1-V3 pattern break, suggesting possible epicardial origin.
Ensuring stable catheter contact and thorough mapping of the periannular region—confirmed by the presence of atrial electrograms—is crucial. Forming a catheter loop enhances mapping accuracy along the mitral annulus, as straightforward transseptal approaches alone may limit sufficient tissue contact.
Following extensive ablation, especially in the LV, transient idioventricular rhythms or increased PVC burden may appear immediately postprocedure, typically indicative of an inflammatory response. These PVCs generally have a lower morphology match compared to clinical PVCs. Rather than pursuing additional ablation, procedure completion and observation may be more appropriate.
Key Tips for Papillary Muscle PVC Ablation
- Utilize ICE guidance extensively
- Consider cryoablation if catheter stability is insufficient
- Verify catheter position carefully with ICE
Conclusion
Implementation of a structured, anatomy-guided workflow significantly improves procedural efficiency and outcomes in PVC ablation. Strategic use of ICE, high-density mapping, and robust lesion validation methods can optimize results, minimize complications, and ensure procedural success.
Disclosures: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Sharma reports consulting fees from Medtronic, Johnson & Johnson MedTech, and Boston Scientific. Dr Bhardwaj reports consulting fees from Biosense Webster, Boston Scientific, and Medtronic, and support for attending meetings and/or travel from Biotronik and Boston Scientific. Dr Garg has no disclosures to report.
Video 1
Video 1. Mapping and ablation of RV septal PVCs. The video demonstrates the real-time electroanatomical mapping and catheter ablation of PVCs originating from the RV septum. The Carto system (Johnson & Johnson MedTech) is used to annotate earliest activation sites based on intracardiac electrograms. Progressive mapping reveals a stable and reproducible local activation time site on the septal wall of the RV.
Video 2
Video 2. Cryoablation of papillary muscle PVCs in ventricular tachycardia (VT). This video captures the cryoablation of an arrhythmia originating from the papillary muscle in a patient presenting with VT and suboptimal contact during prior RF ablation.
References
- Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019;21(8):1143-1144. doi:10.1093/europace/euz132
- Sousa PA, Tonko J, Dilling-Boer D, et al. Real-world assessment of multipolar and point-by-point mapping for premature ventricular contraction ablation. Europace. 2024;26(6):euae148. doi:10.1093/europace/euae148
- Pang Y, Xu Y, Cheng K, et al. A new insight into the anatomical ablation approach at R-L ILT for VAs with a left ventricular summit origination: electrophysiological characteristics and catheter ablation. J Interv Card Electrophysiol. 2025;68(3):709-720. doi:10.1007/s10840-024-01974-w
- Enriquez A, Muser D, Markman TM, Garcia F. Mapping and ablation of premature ventricular complexes: state of the art. JACC Clin Electrophysiol. 2024;10:1206-1222. doi:10.1016/j.jacep.2024.02.00
- Gordon JP, Liang JJ, Pathak RK, et al. Percutaneous cryoablation for papillary muscle ventricular arrhythmias after failed radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2018;29(12):1654-1663. doi:10.1111/jce.13716z











