Redefining FARAPULSE™ Mapping Integration: Harnessing the Power of the OPAL HDx™ Mapping System
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of EP Lab Digest or HMP Global, their employees, and affiliates.
EP LAB DIGEST. 2025;25(7):8-9.
Jason Meyers, MD, Iowa Heart Center, West Des Moines, Iowa
Contributor: Justin Kline, BS, RN, Senior Mapping Specialist, Boston Scientific Corporation
In the evolving field of electrophysiology, clarity and efficiency are paramount. At MercyOne Iowa Heart Center, we have been early adopters of the FARAPULSE™ PFA Platform (Boston Scientific), and the integration of the FARAVIEW™ Software Module on the OPAL HDx™ Mapping System has markedly changed the way we approach pulmonary vein isolation (PVI). This integrated mapping solution bridges the gap from traditional high-density mapping and allows real-time visualization. This, combined with VersaCross Connect™ Access Solution for FARADRIVE™, provides an extremely efficient workflow with no exchanges. This is done without compromising safety or efficacy.
FARAVIEW integrates electroanatomic mapping with data collected directly from the navigation-enabled catheter, a capability that, until now, was not possible. This means we no longer rely exclusively on high-density mapping catheters to assess lesion sets—we can now map, ablate, and validate with a single tool. This streamlined workflow has tremendous implications for case efficiency, while maintaining procedural confidence.
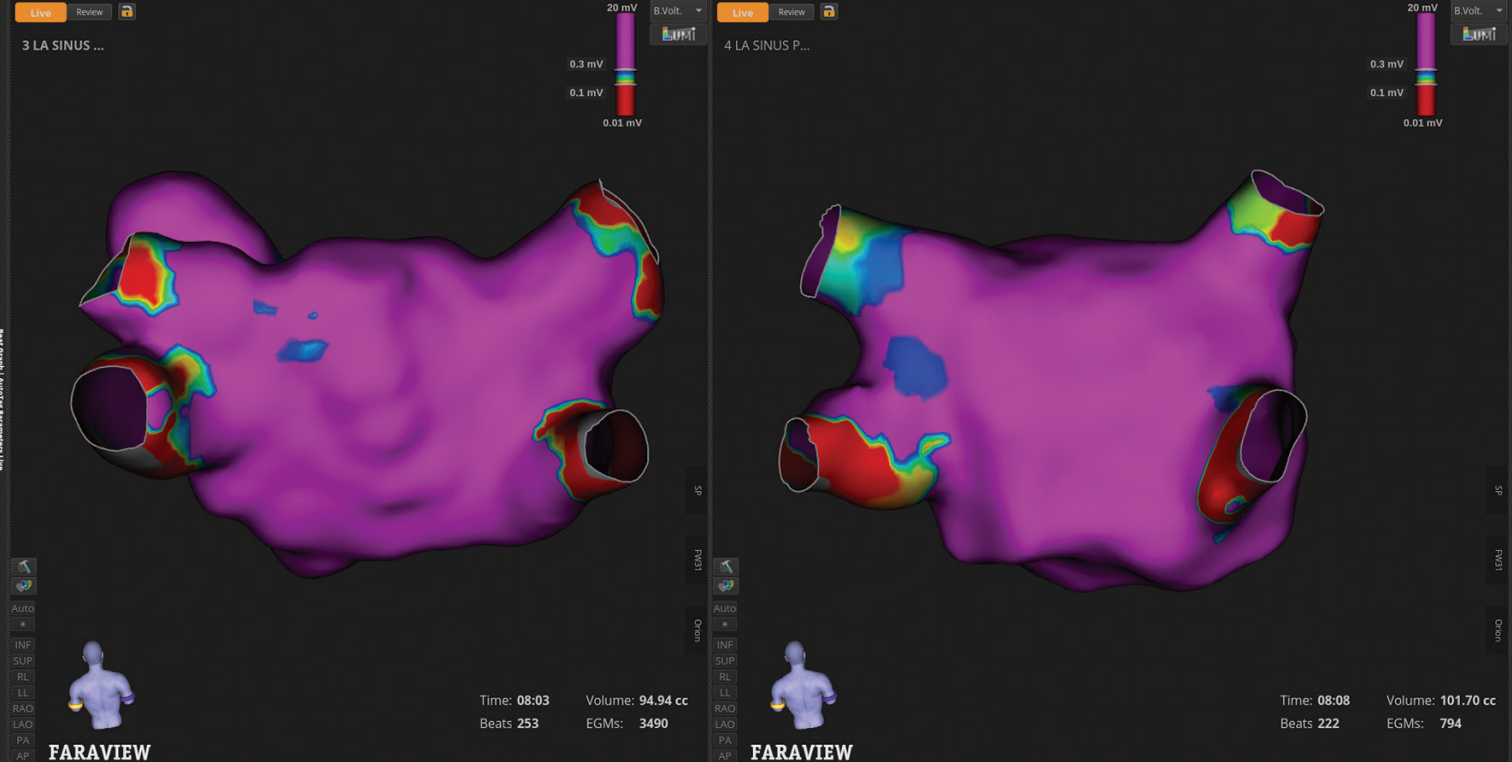
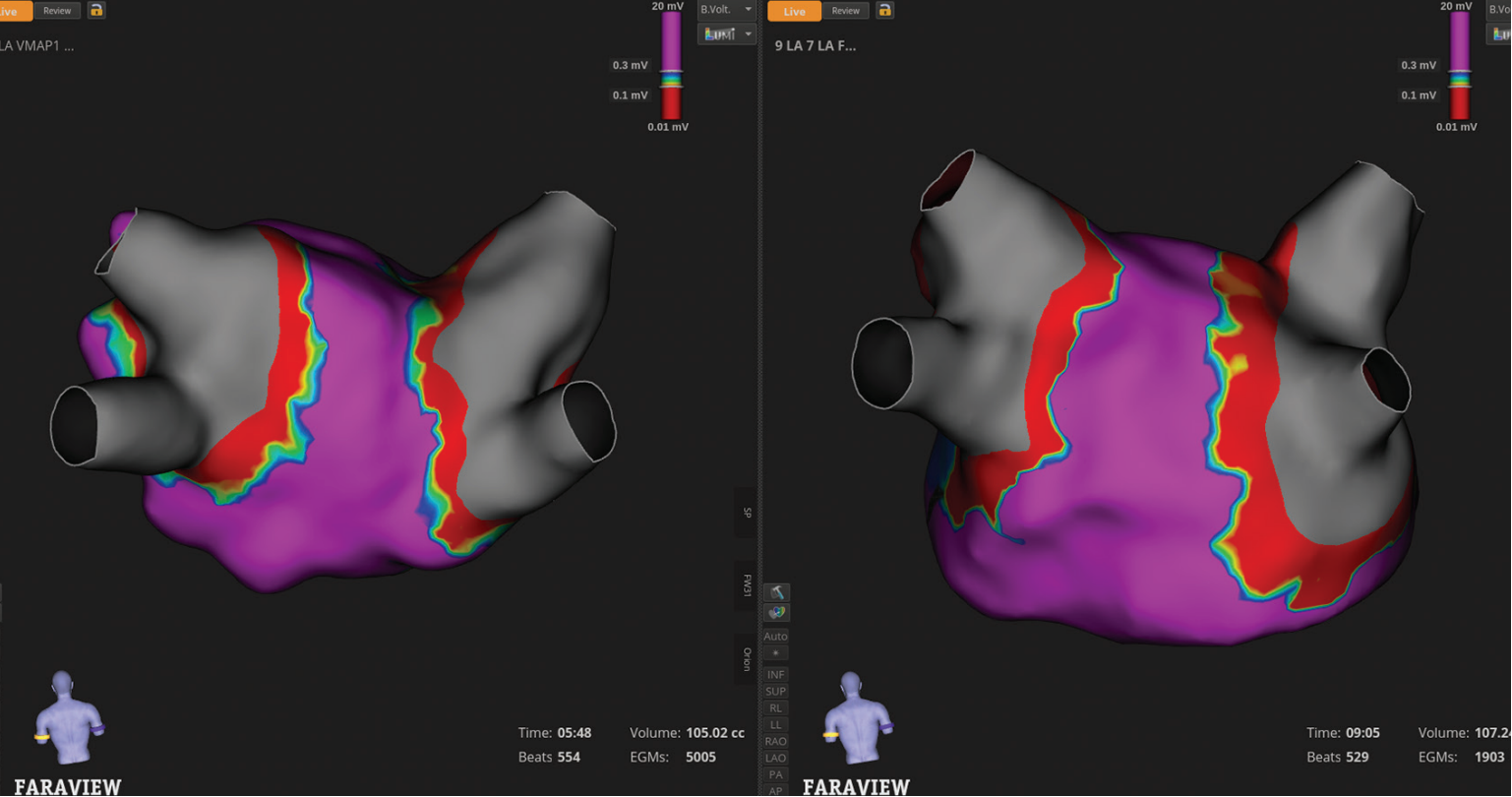
This case includes several mapping images that demonstrate the value of the enhanced FARAPULSE mapping experience with FARAVIEW on OPAL HDx. One key comparison is between pre- and post-ablation maps created with the high-definition (HD) INTELLAMAP ORION™ Mapping Catheter, and the same map generated with the FARAWAVE™ NAV PFA Catheter (Figures 1 and 2). While the ORION map has much higher point density, the FARAWAVE NAV-derived map shows striking consistency in substrate voltage.
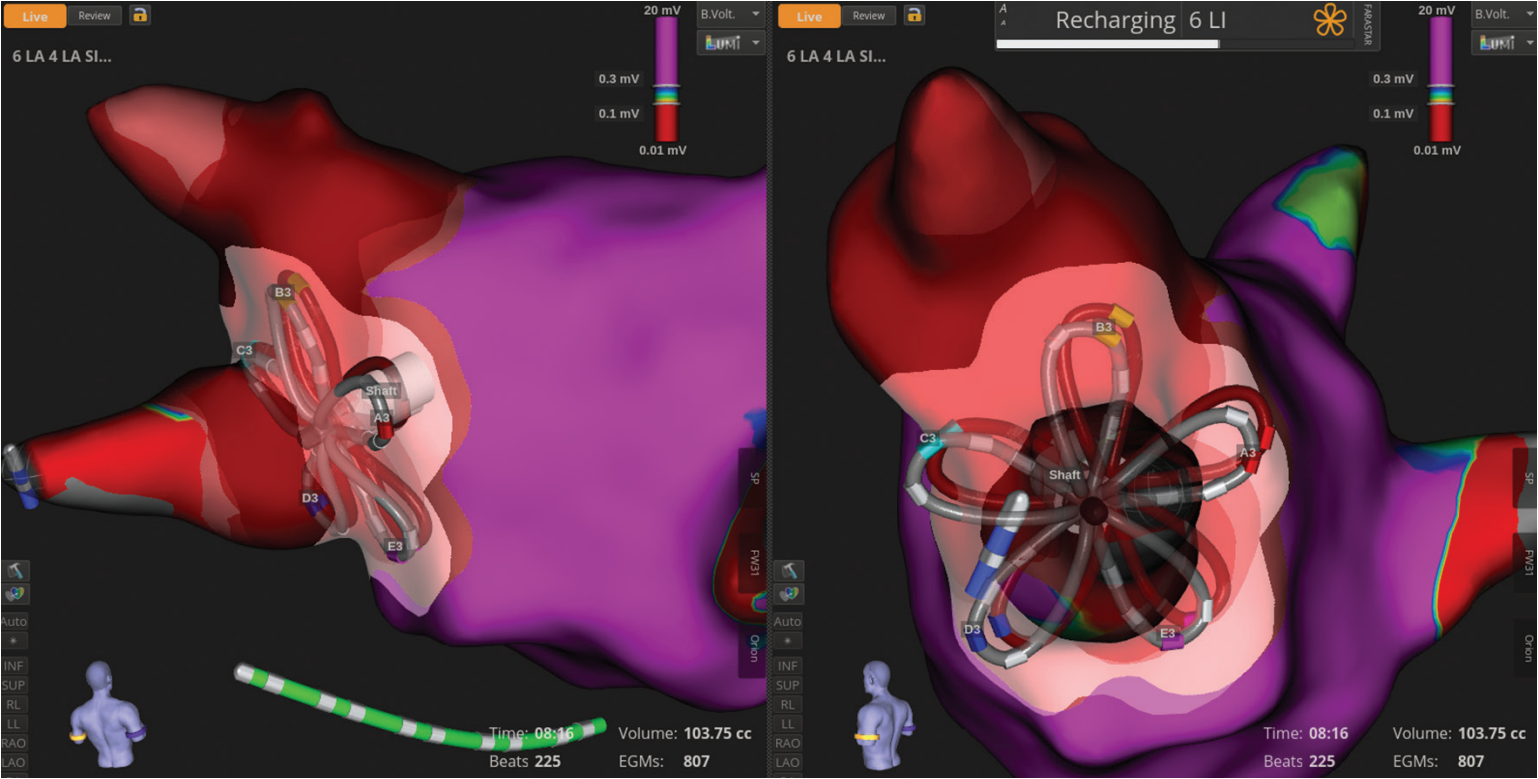
FARAVIEW’s FIELDTAG™ Technology is a key strength of the system. This advanced tagging tool offers automated, visual insight into ablation location and overlap, giving greater confidence in therapy delivery (Figure 3). It enables quick assessment of potential gaps in ablations and supports a simplified, reproducible approach for all of our PVI patients. Additionally, the ablation preview tool allows for visualization and planning of lesions before delivery, ensuring high precision and clear understanding of lesion volume prior to application.
In terms of lab efficiency, we can reduce catheter exchanges and eliminate the need for a second mapping system during FARAPULSE cases. This translates to shorter procedure times and less complexity for both staff and patients. For a high-volume center like ours, the cumulative time savings can be significant. Since we have started using the FARAPULSE PFA Platform to treat our PVI patients, we have significantly reduced our backlog of patients and shortened their time to treatment. This has reduced our wait time for atrial fibrillation ablation from 5 to 6 months down to 1 to 2 months.
Disclosures: Dr Meyers and Mr Kline have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Meyers reports support for the present manuscript from Boston Scientific; he also reports consulting fees and payment or honoraria for presentations from Boston Scientific. Mr Kline reports that he is an employee of Boston Scientific.
This content was published with support from Boston Scientific.











