Diagnostic and Procedural Advantages of a Single-Chamber Implantable Cardioverter-Defibrillator With Atrial Sensing Capability: Two Case Studies
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of EP Lab Digest or HMP Global, their employees, and affiliates.
EP LAB DIGEST. 2025;25(6):ONLINE ONLY.
Karthik Venkatesh Prasad, MD
North Mississippi Medical Center, Tupelo, Mississippi
Present and permanent address where the work as done:
North Mississippi Medical Center
830 S. Gloster Street, Tupelo, MS 38801 (USA)
Introduction
The first generation of cardiac implantable electronic devices (CIEDs) with a single-pass lead entered the market in the 1980s, but unreliable sensing ultimately resulted in the gradual abandonment of these devices. Significant improvements in atrial sensing amplification, P-wave stabilization, signal filtration, and lead design were introduced in a family of iterative devices of the DX ICD family (BIOTRONIK).1,2 Enhancements included optimized atrial dipole spacing and placement, improved signal processing increasing atrial gain fourfold, and a wider bandpass for atrial sensing, all of which allow for greater flexibility in terms of lead placement.2 However, these DX devices do not offer dual-chamber pacing.2
The decision for implantation of ICDs per guideline recommendations compel the clinician to consider cardiac structural anomalies and the nature of the patient’s underlying cardiac diagnosis as well as presence of arrhythmias, any of which may complicate therapy. While millions of patients have benefited from potentially lifesaving ICD therapy, not all devices are suitable for all patients. These 2 case reports demonstrate the appropriate use of the BIOTRONIK DX ICDs to effectively manage 2 different and clinically challenging scenarios.
Case Report #1
A 50-year-old woman with dilated nonischemic cardiomyopathy and chronic systolic heart failure had a BIOTRONIK DX ICD (Acticor VR-T DX DF4 ProMRI, BIOTRONIK) implanted for primary prevention of sudden cardiac death in July 2021. Eighteen months post implant, she presented to the emergency department (ED) with recurrent episodes of sudden-onset syncope. Evaluation in the ED showed stable hemodynamics with an electrocardiogram (ECG) showing normal sinus rhythm.
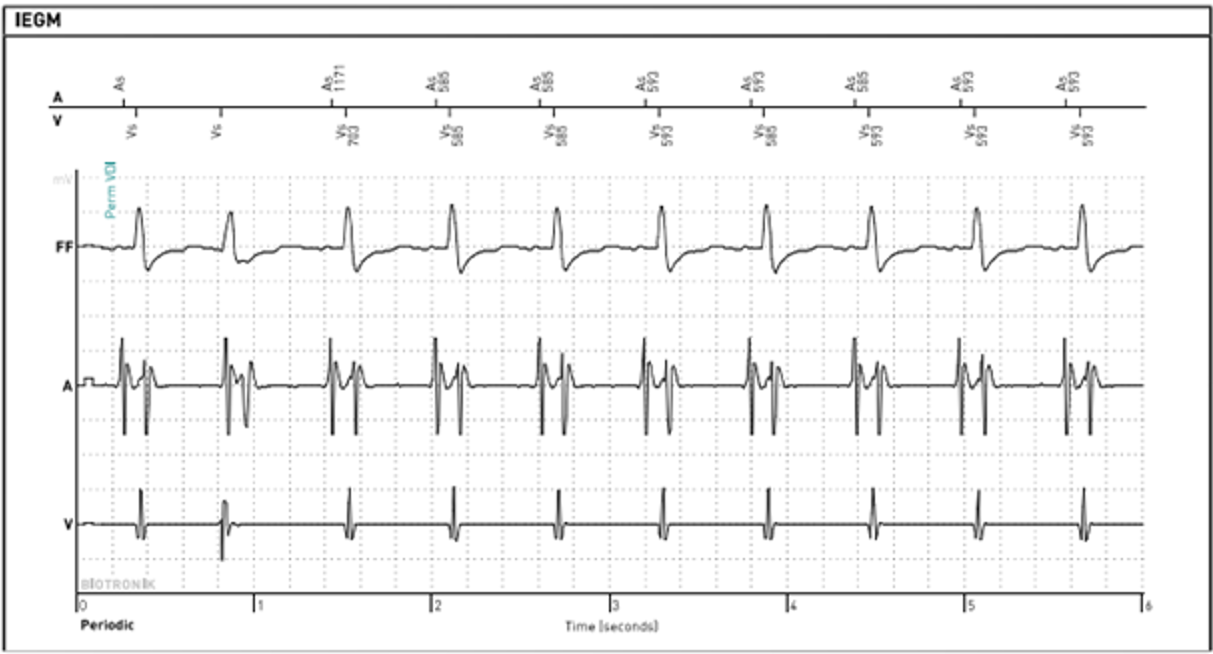
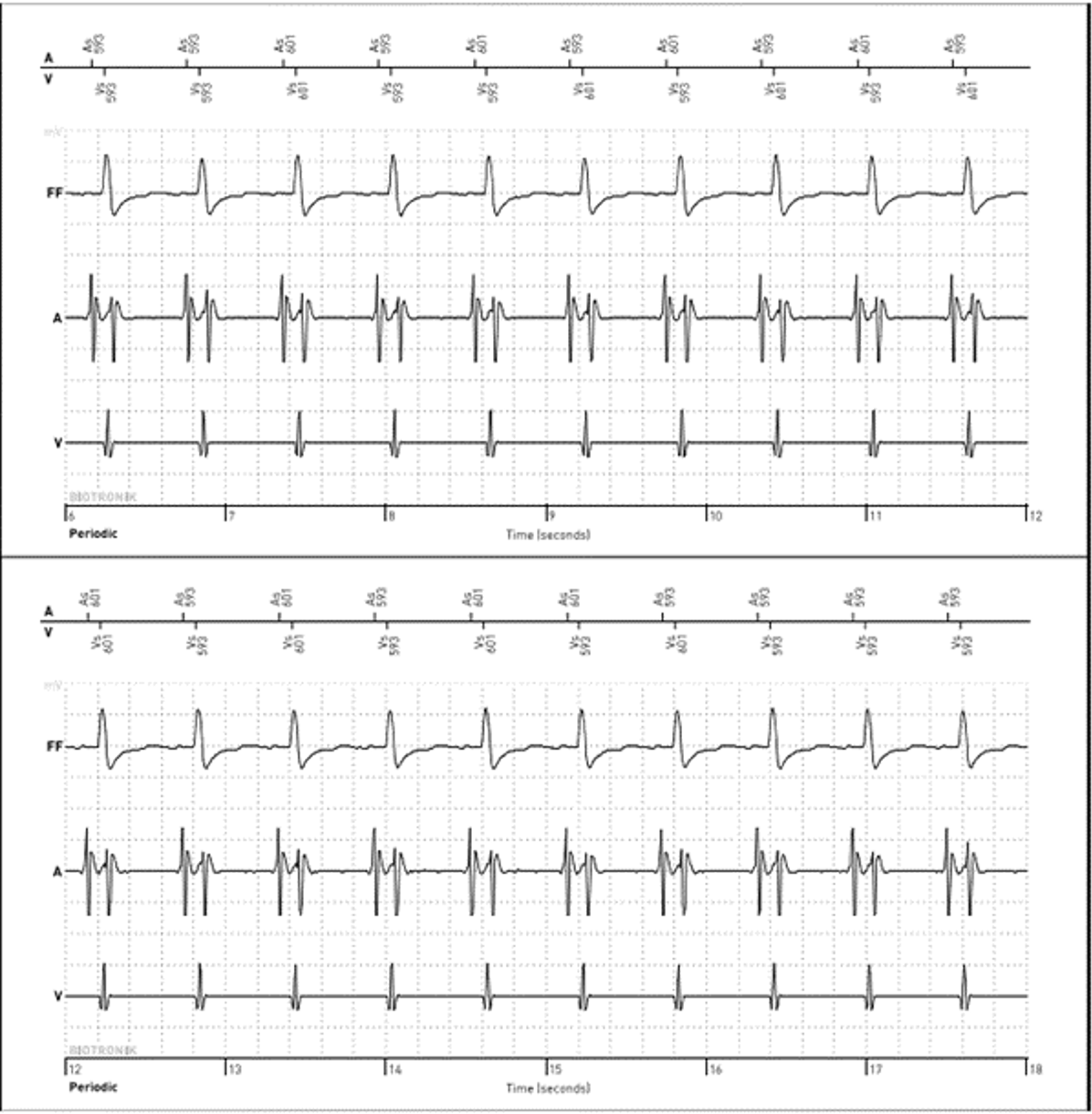
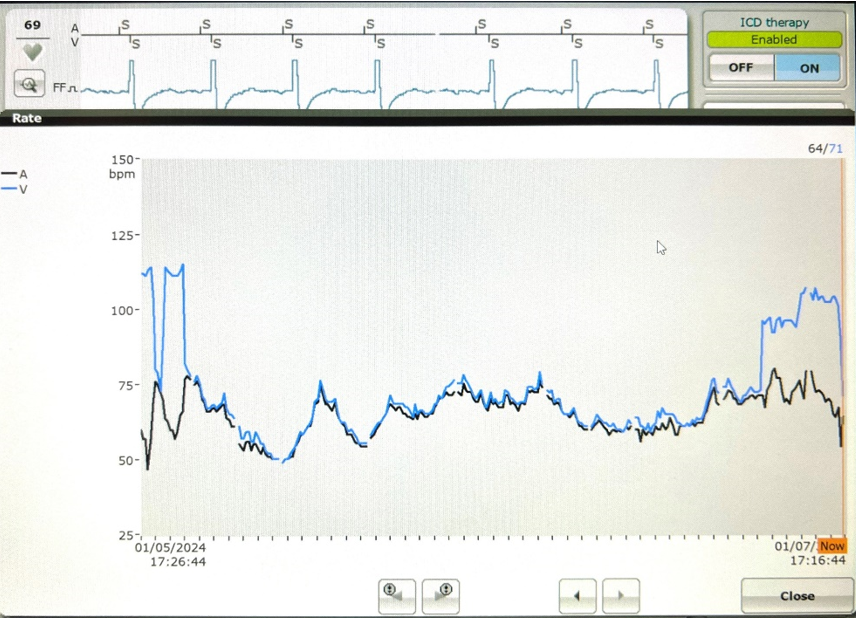
Initial interrogation of the patient’s ICD showed no alerts for ventricular or atrial tachyarrhythmias. Ventricular pacing was 0% at a lower rate of 40 beats per minute (bpm), suggesting no underlying profound bradycardia or pauses to explain the syncope. A review of the preceding 48 hours of the heart rate trend showed that immediately prior to coming to the ED, the atrial and ventricular rates on the diagnostic reports had separated; the atrial rate remained consistent 60-70 bpm; however, the ventricular rate was approximately 100-110 bpm (Figure 1).
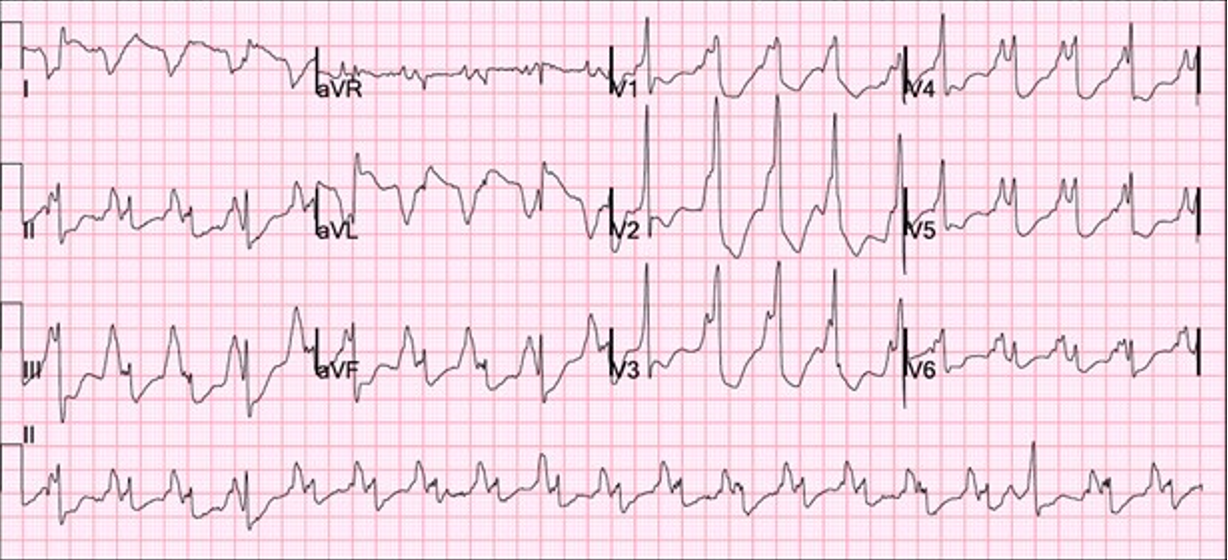
This suggested the possibility of slow ventricular tachycardia (VT) as the potential etiology of the syncope. She was admitted to the hospital for further management. The next morning, the patient experienced an episode of near-syncope and telemetry monitoring picked up wide-complex tachycardia. An immediate ECG was obtained as shown below (Appendix). A subsequent interrogation of the DX ICD also confirmed the diagnosis of ventricular tachycardia.
Antiarrhythmic drugs and catheter ablation were discussed as treatment options. Given her young age and the potential feasibility of mapping and ablating the slow VT, a decision was made to attempt catheter ablation of the VT.
Intraoperatively, clinical VT was induced with pacing maneuvers. Endocardial and percutaneous epicardial maps of the left ventricle (LV) were performed based on the VT morphology and substrate. Under general anesthesia, VT was not sustainable but was repeatedly inducible. Hence, pace mapping was performed to identify the substrate/exit for the clinical VT. Pace mapping on the basal-mid antero-lateral epicardial surface of the LV showed a nearly identical (97%) match to the clinical VT (Figure 2), identifying the exit site.
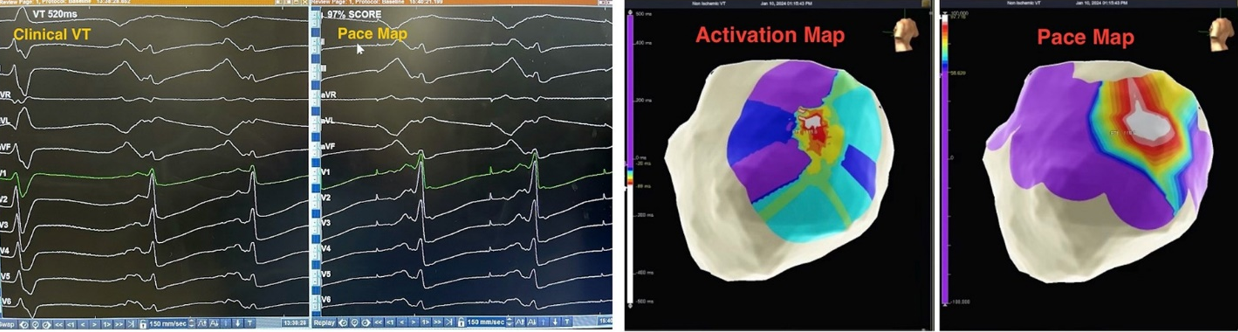
Radiofrequency ablation on the epicardial LV (ensuring a safe distance from nearby epicardial coronary arteries on angiogram) rendered clinical VT non-inducible. The patient required vasopressors during the procedure due to systolic heart failure and use of general anesthesia. The procedure was otherwise uneventful with no immediate complications noted.
Slow VT with a rate below the traditional programmed VT zone is easily missed on single-chamber ICDs; however, the atrial sensor on the single-chamber DX ICD lead provided information leading to the successful diagnosis and treatment of slow VT.
Case Report #2
A 65-year-old woman with coronary artery disease requiring prior coronary artery bypass surgery, as well as ischemic cardiomyopathy with a LV systolic ejection fraction <30%, was referred for ICD implantation for primary prevention of sudden cardiac death. She had New York Heart Association Class II-III chronic systolic heart failure and was being treated with guideline-directed medical therapy. The ECG showed normal sinus rhythm with a QRS duration of <100 milliseconds. There was no prior diagnosis of atrial fibrillation (AF), supraventricular tachycardia, or sinus node dysfunction. Based on this history, it was decided to implant a DX ICD (Acticor VR-T DX DF4 ProMRI).
The outpatient procedure was performed under conscious sedation in the electrophysiology lab. Intraoperatively, a standard incision was made in the left delto-pectoral groove and access into the left axillary vein was obtained using ultrasound guidance. A J-tipped wire was advanced under fluoroscopy. At this point, it was evident that the wire did not cross the midline of the chest to enter the superior vena cava, but rather passed from the left subclavian vein into a persistent left superior vena cava (PLSVC) into the coronary sinus (CS) and then into the right atrium (RA). This was confirmed on right and left anterior oblique views. An access sheath was advanced and a DX ICD lead (Pamira S DX 64/15, 15 cm spacing from lead tip to dipole) was advanced via the sheath, into the PLSVC, CS, and into the RA. Using a manually shaped stylet, the lead was prolapsed across the tricuspid valve into the right ventricle (RV) and the helix deployed. Testing of the lead showed appropriate sensing, pacing threshold, and impedance. The atrial sensing dipoles were on the floor of the CS with appropriate P-wave sensing. The sheath was split, and the lead was secured and connected to the device. Repeated testing showed proper sensing of both atrial and ventricular signals (far-field sensing of the ventricle was detected by the atrial dipoles but appropriately labeled by the device). The procedure was completed in under one hour. A postoperative chest x-ray was performed in 2 views (Figure 3).
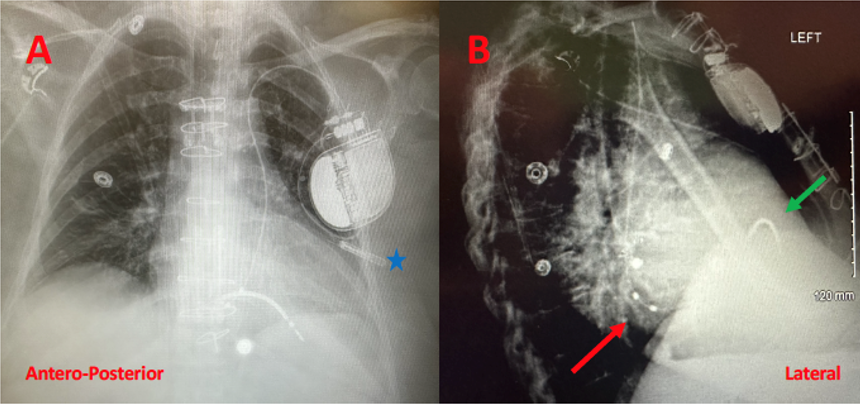
After 4 hours of observation, the patient was discharged home the same day with no complications noted. The 1-week postoperative visit was normal and remote monitoring has been activated. A sample of the device interrogation 3 months after implant appears in the Appendix.
Persistent left superior vena cava is the most common congenital abnormality of the thoracic venous return system, affecting an estimated incidence of 0.3%-0.5% of the general population. Most patients have a normal right-sided SVC as well. Presence of PLSVC is not a contraindication for cardiac device implant (pacemakers or defibrillators), but it may challenge conventional implant techniques. This case demonstrates how the single-lead DX ICD wire can provide both atrial and ventricular electrograms even in an unusual anatomy.
Discussion
Despite the numerous advantages offered by a single-lead ICD system—including lower risk of complications, less hardware in the body, lower costs, and shorter procedure times3—dual-chamber ICDs continue to be widely used, even in patients without an atrial pacing indication. The DX ICD systems have advanced device-based algorithms which allow for atrial sensing, rhythm discrimination, and atrial electrograms in a single-lead system. Subclinical AF in the form of atrial high-rate activity was accurately detected significantly more often in DX device patients than patients with a conventional single-chamber system and at the same rate as conventional dual-chamber patients, with no instances of inappropriate therapy in the DX group.4 In a 2-year study of 1841 DX ICD patients, atrial high-rate episodes of 1 hour or more were detected with 99.7% accuracy.5
Thus, a single-lead device has an established utility for many patients, but selecting the appropriate device for any given patient requires clinical judgment. VT at rates below standard programming zones can be a clinical challenge because the arrhythmia can go undetected even when it causes symptoms. Such accelerated idioventricular rhythms may be associated with symptoms such as palpitations, chest discomfort, syncope, and exercise intolerance.6 Device-based algorithms are often helpful in diagnosing slow VT, as other case reports have shown.7
In the first case, the DX ICD demonstrated upon interrogation that the atrial and ventricular rates had abruptly dissociated, something which would not have been apparent in a conventional single-chamber ICD. ECG and telemetry monitoring confirmed the diagnosis. The DX ICD was able to sense both atrial and ventricular activity independently through a single lead fixated in the RV with floating electrode in the atrial blood pool to sense atrial activity. While slow VT is generally considered stable and benign, it can cause debilitating symptoms and may become persistent.8 In this case, device diagnostics allowed for a relatively rapid diagnosis, leading to timely and appropriate therapeutic intervention.
The second case described the implant of an ICD in a patient with a venous anomaly, PLSVC. Typically, this finding is not clinically significant in terms of hemodynamics, but its incidental discovery imposed important procedural considerations for device implant as well as imaging challenges.9,10 The literature reports successful single-lead implantation of a VDD system in a patient with PLSVC with successful atrial and ventricular sensing.11
This PLSVC drained into the RA by way of a dilated CS. In this setting, placing a defibrillation lead presents some technical challenges to the implanting physician. It was imperative that the atrial dipoles of the DX ICD lead be properly situated in the RA for optimal device performance. An alternative approach would be to implant the device in the right chest, after ensuring the presence of a right-sided SVC, possibly utilizing a venogram via a right arm intravenous line. In this case, considering an incision and ICD pocket were already created on the left chest, it was decided to proceed with ICD implant via the PLSVC.
This report has certain limitations. These 2 cases are from a single center and were performed by the same physician with considerable experience implanting these devices, which may limit their generalizability.
Conclusion
The DX ICD system of today offers reliable atrial sensing and diagnostics via floating atrial dipoles, and a proprietary amplification algorithm. These devices performed well in 2 complex and atypical ICD cases, one involving a slow VT and the other a venous/structural anomaly. This technology has demonstrated specific advantages such as reliable atrial diagnostics, a minimized number of implanted leads and lead-related complications (eg, dislodgement, lead failure, and vein occlusion), and the ease of a single-lead system that provides dual-chamber sensing.
Disclosure: Dr Prasad has completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The author reports consulting honoraria from BIOTRONIK. The author has not received any financial support for this case study manuscript.
This content was published with support from BIOTRONIK.
References
1. Powers EM, Mazur A. Editorial commentary: VDD ICD systems: can they keep us afloat in a sea of dual chamber devices? Trends Cardiovasc Med. 2022;32(2):90-91. doi:10.1016/j.tcm.2021.01.008
2. Vamos M, Nemeth M, Balazs T, Zima E, Duray GZ. Rationale and feasibility of the atrioventricular single-lead ICD systems with a floating atrial dipole (DX) in clinical practice. Trends Cardiovasc Med. 2022;32(2):84-89. doi:10.1016/j.tcm.2021.01.003
3. Margolis G, Hamuda N, Kobo O, et al. Single- versus dual-chamber implantable cardioverter-defibrillator for primary prevention of sudden cardiac death in the United States. J Am Heart Assoc. 2023;12(15):e029126. doi:10.1161/jaha.122.029126
4. Thomas G, Choi DY, Doppalapudi H, et al. Subclinical atrial fibrillation detection with a floating atrial sensing dipole in single lead implantable cardioverter-defibrillator systems: results of the SENSE trial. J Cardiovasc Electrophysiol. 2019;30(10):1994-2001. doi:10.1111/jce.14081
5. Hindricks G, Theuns DA, Bar-Lev D, et al. Ability to remotely monitor atrial high-rate episodes using a single-chamber implantable cardioverter-defibrillator with a floating atrial sensing dipole. Europace. 2023;25(5):euad061. doi:10.1093/europace/euad061
6. Wang L, Liu H, Zhu C, et al. Clinical characteristics and therapeutic strategy of frequent accelerated idioventricular rhythm. BMC Cardiovasc Disord. 2021;21(1):425. doi:10.1186/s12872-021-02221-0
7. Barold SSD, Kucher AD. Diagnosis of slow ventricular tachycardia by cardiac resynchronization devices utilizing a desynchronization detection algorithm. J Electrocardiol. 2020;59:140-146. doi:10.1016/j.jelectrocard.2020.02.007
8. Errahmouni A, Bun SS, Latcu DG, Tazi-Mezalek A, Saoudi N. Accelerated idioventricular rhythm requiring catheter ablation in a child: the dark side of a benign arrhythmia. Ann Cardiol Angeiol (Paris). 2017;66(5):323-325. doi:10.1016/j.ancard.2017.09.002
9. Dave V, Sesham K, Mehra S, Roy TS, Ahuja MS. Persistent left superior vena cava: an anatomical variation. Med J Armed Forces India. 2022;78(Suppl 1):S277-s281. doi:10.1016/j.mjafi.2020.01.009
10. Azizova A, Onder O, Arslan S, Ardali S, Hazirolan T. Persistent left superior vena cava: clinical importance and differential diagnoses. Insights Imaging. 2020;11(1):110. doi:10.1186/s13244-020-00906-2
11. Vamos M, Saghy L, Bencsik G. Implantation of a VDD implantable cardioverter-defibrillator lead via a persistent left superior vena cava. Herzschrittmacherther Elektrophysiol. 2022;33(1):81-83. doi:10.1007/s00399-021-00835-7