Contact Matters in Pulsed Field Ablation: Streamlining FARAPULSE™ PFA Workflows With Contact Sensing on OPAL HDx™
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of EP Lab Digest or HMP Global, their employees, and affiliates.
EP LAB DIGEST. 2025;25(12):7.
David S Frankel, MD, and Thomas A Boyle, MD
Hospital of University of Pennsylvania, Philadelphia, Pennsylvania
The tissue selectivity of pulsed field ablation (PFA) has not eliminated the need for excellent tissue contact during atrial fibrillation ablation. Adequate contact minimizes gaps, ensures durable lesion formation, and may reduce hemolysis caused by poorly opposed electrodes. After adopting PFA, many operators in our high-volume practice have incorporated left atrial (LA) intracardiac echocardiography (ICE) into their workflows to visualize catheter contact in real time, compensating for the loss of contact-force sensing catheters that were standard in radiofrequency ablation.
Recently, impedance-based contact sensing was introduced as an additional tool for real-time contact assessment of the FARAWAVE™ NAV Catheter (FARAPULSE™ PFA Platform, Boston Scientific). In this case, we describe a representative workflow using the FARAWAVE NAV Catheter integrated with the OPAL HDx™ Mapping System (Boston Scientific), highlighting how contact sensing aids mapping and ablation.
After advancing to the right atrium (RA), transseptal puncture was performed using the VersaCross Connect™ Access Solution and FARADRIVE™ Steerable Sheath (Boston Scientific) under fluoroscopic and ICE guidance. The sheath and dilator were then pulled back into the RA, leaving the pigtail wire across the fossa ovalis. Using the wire as a guide, the ICE catheter was advanced across the fossa ovalis into the LA. The FARADRIVE Sheath was then readvanced into the LA. The FARAWAVE NAV Catheter was inserted and opened in the flower configuration, then spun freely in the blood pool to calibrate baseline impedance across E3 electrodes (Figure 1). These measurements establish the reference for real-time detection of tissue contact. A baseline voltage map was obtained using the FARAWAVE NAV Catheter and OPAL HDx.
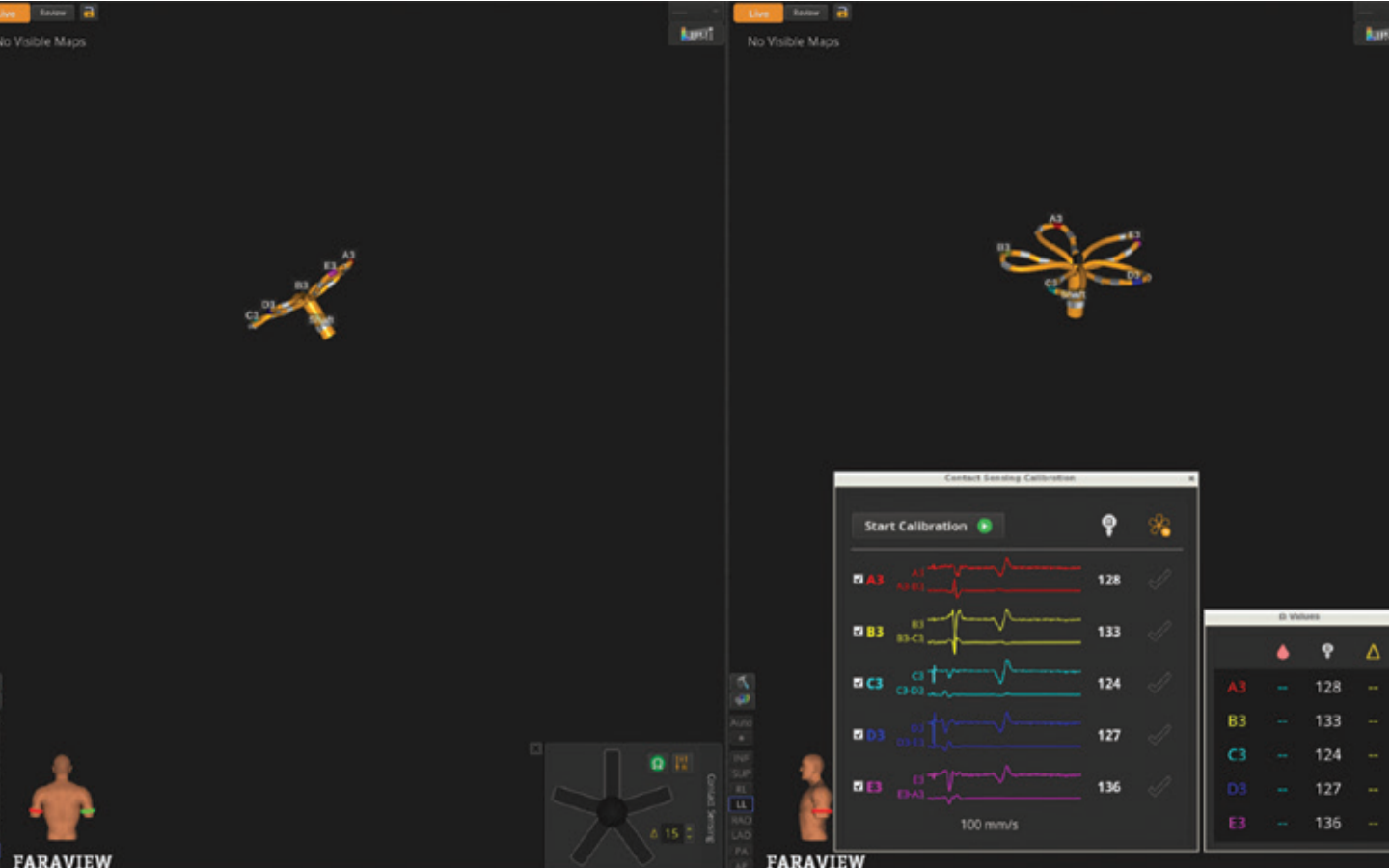
Contact sensing utilizes local impedance measurements from the third electrode on each catheter spline. Impedance changes of approximately 15 ohms in the vein antra and 10 ohms along the posterior wall were used to trigger electrode illumination on the map, helping to identify areas of adequate contact and potential gaps before energy delivery. If desired, operators can view quantitative impedance changes for each electrode (Figure 2).
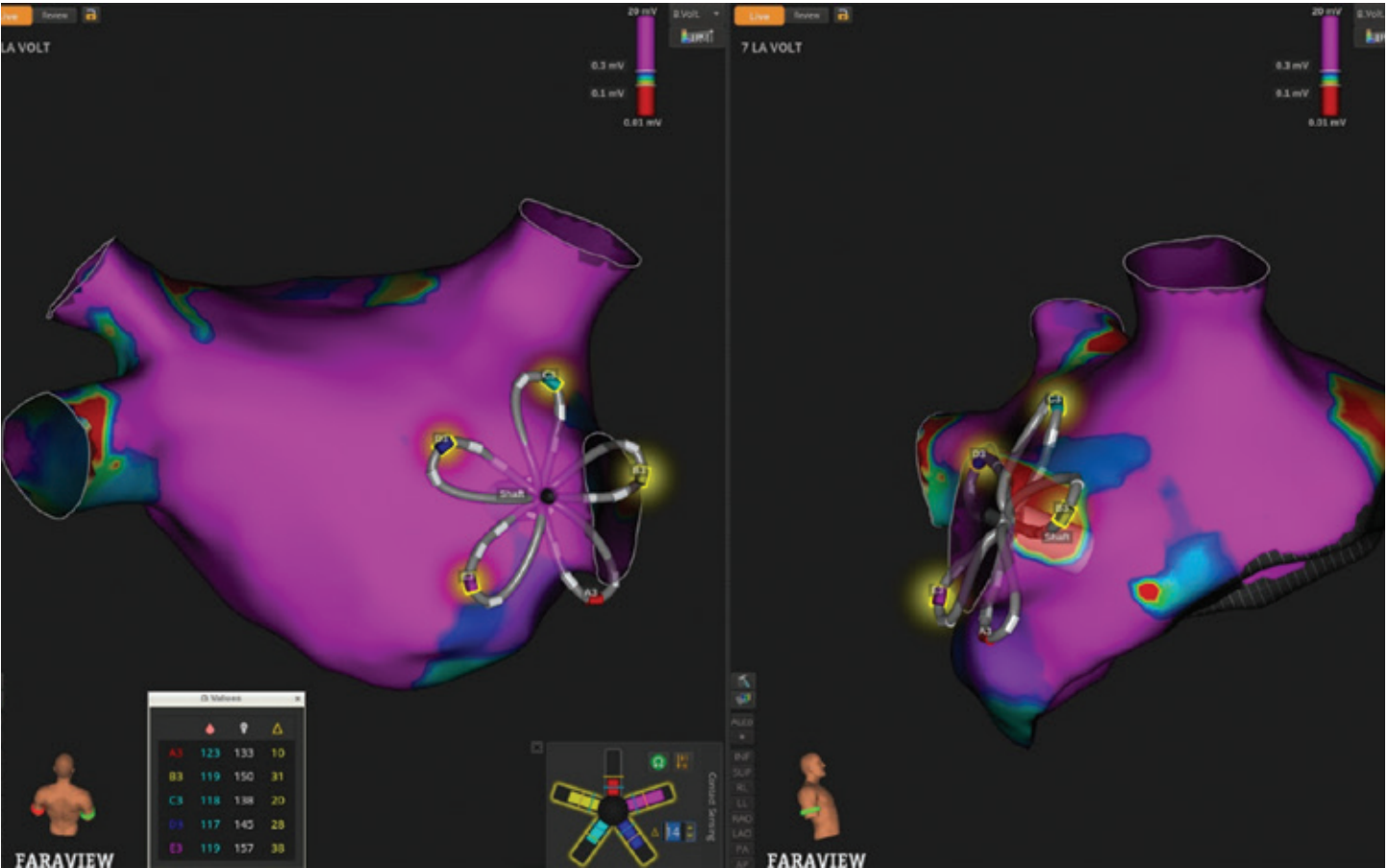
As the catheter was maneuvered around each pulmonary vein (PV), the system displayed real-time electrode configuration and petal deflection, allowing direct correlation between mechanical deformation and tissue engagement, which we were able to corroborate with LA ICE and contact sensing. As visualizing the contact status of all 5 petals simultaneously can be challenging with a single ICE view, contact sensing provides distinct and complementary information to streamline contact assessment. Projected PFA markers (FIELDTAG™ Technology, Boston Scientific) were displayed to indicate anticipated ablation zones, providing a continuous visual guide to ensure circumferential coverage. Ablation was then performed without exchanging catheters or introducing additional sheaths, maintaining a streamlined workflow.
Following completion of PV isolation, the same FARAWAVE NAV Catheter was used to immediately remap the LA. Post-ablation voltage mapping along the posterior wall again leveraged impedance-based contact visualization (Figure 3), confirming consistent tissue engagement and supporting assessment of lesion completeness.
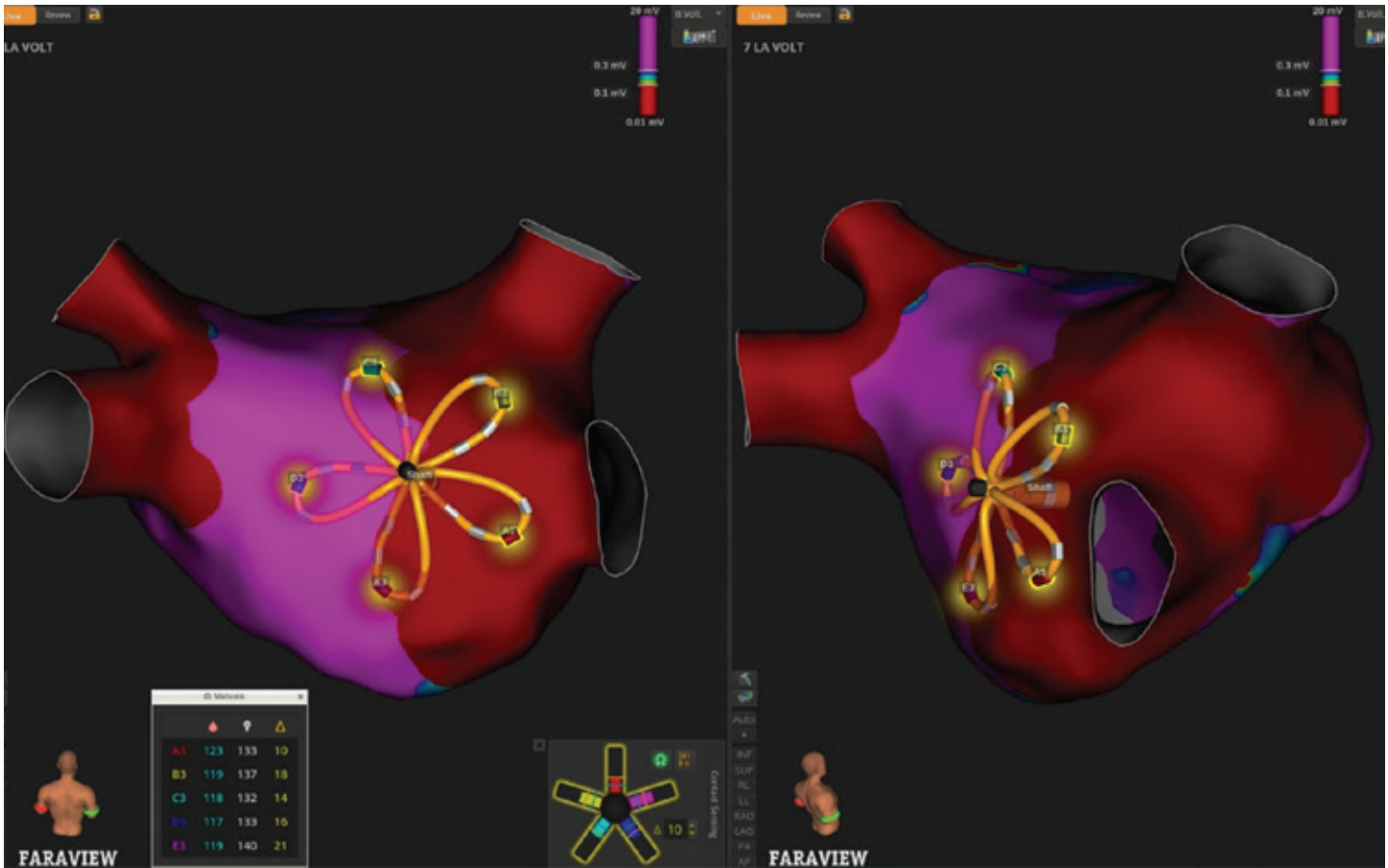
In summary, even in the context of PFA, careful attention to tissue contact remains a key determinant of procedural success. This FARAPULSE PFA workflow demonstrates that impedance-calibrated contact sensing allows integration of mapping, ablation, and post-procedural evaluation into a single, continuous process, which may reduce procedural time, fluoroscopy, and other adjunctive imaging.
Disclosures: Drs Frankel and Boyle have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Drs Frankel and Boyle report support for the present manuscript from Boston Scientific.
This content was published with support from Boston Scientific.











