A Functional Approach to Ventricular Tachycardia Ablation in Surgically Repaired Tetralogy of Fallot
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of EP Lab Digest or HMP Global, their employees, and affiliates.
EP LAB DIGEST. 2025;25(8):8-10.
James Russell, DO1; Richard Amoateng, MD1; Dana Johnson, MD1; Adarsh Bhan, MD2; Frank Zimmerman, MD2
1University of Illinois at Chicago, Chicago, Illinois; 2Advocate Christ Medical Center, Oak Lawn, Illinois
Case Presentation
A 41-year-old man with a past medical history of tetralogy-type double outlet right ventricle (DORV) with surgical repair at 2 years old, atrial fibrillation (AF), surgical pulmonary valve replacement with Maze procedure 10 years prior, transcatheter pulmonary valve-in-valve replacement 4 months prior, ventricular fibrillation episode and secondary prevention dual-chamber implantable cardioverter-defibrillator (ICD) 10 years prior, who presented for ventricular tachycardia (VT). He had multiple episodes of monomorphic VT treated with antitachycardia pacing, resulting in a type-2 break after converting to short salvos of polymorphic VT followed by sinus rhythm. Dofetilide had been started previously for persistent AF and atrial tachycardias despite the prior Maze procedure. The patient did not want a change or escalation in antiarrhythmic therapy, so they elected for an electrophysiology (EP) study and catheter ablation of VT.
Surgical History
The surgical repair for his tetralogy-type DORV was performed at 2 years old, which included transventricular resection of the right ventricular infundibular muscle, Dacron patch ventricular septal defect (VSD) closure, and pulmonary valvotomy. Due to severe pulmonary insufficiency, he underwent a surgical bioprosthetic valve replacement and a Maze procedure 10 years prior to presentation. He developed significant right heart failure due to severe bioprosthetic pulmonary insufficiency. A transcatheter valve-in-valve procedure known as transcatheter pulmonary valve replacement (TPVR) was performed successfully with the improvement of his symptoms; however, 1 month later, he developed recurrent ventricular arrhythmias requiring antitachycardia pacing.
Preprocedural Evaluation
A complete panel of blood work including complete blood count, comprehensive metabolic panel, and thyroid studies were within a normal range. An echocardiogram was performed demonstrating a left ventricular ejection fraction of 50% to 55%, moderately depressed RV function with severe dilation, no residual VSD at the site of repair, and mild perivalvular pulmonary valve-in-valve leak. A coronary angiogram demonstrated no anomalous coronary artery insertions and normal vessels.
EP Procedural Methods
The patient was brought to the EP lab in a fasting state and placed under general anesthesia. Venous and arterial access were obtained. The Carto 3 system (Johnson & Johnson MedTech) was used to facilitate 3-dimensional mapping with integration of intracardiac echocardiography. Mapping was performed using the Optrell catheter (Johnson & Johnson MedTech) during RV pacing and VT. Ablation was performed with the ThermoCool SmartTouch SF catheter (Johnson & Johnson MedTech) using 35W to 45W for 1 to 2 minutes. A standard VT stimulation protocol with up to 3 extrastimuli from 2 sites on and off of isoproterenol was used at the beginning and end of the case.
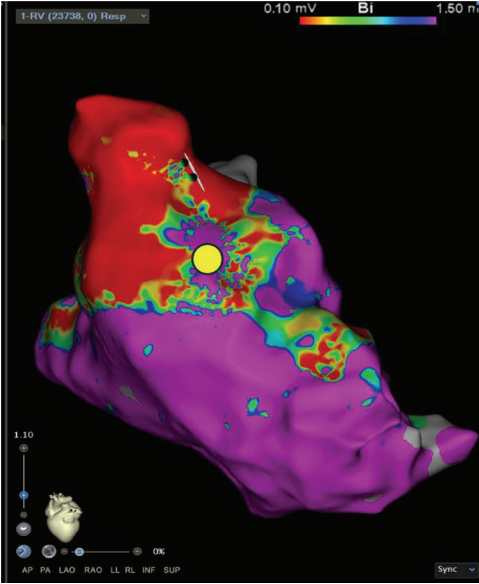
Substrate Mapping
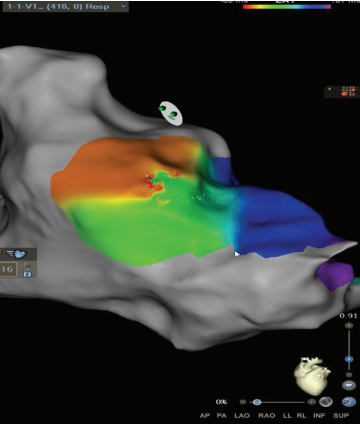
A high-definition map was constructed with an Optrell catheter and RV pacing to better understand the patient’s surgical scar-based substrate before ventricular stimulation (Figure 1). The scar was noted almost exclusively in the RV outflow tract anteriorly stretching across to the tricuspid valve annulus. A small amount of patchy scar was noted in the anterior mid to apical RV as well; however, the septum did not have a significant scar near the predicted VSD Dacron patch. A slowly conducting anatomic isthmus (SCAI) with a conduction velocity of <0.5 m/s was marked (yellow circle, Figure 1). The conduction velocity cutoff of <0.5 m/s has been predictive of arrhythmogenic isthmuses in repaired Tetralogy of Fallot (rTOF).1,2 Preemptive ablation of identified SCAIs is associated with lower rates of VT and ICD implantations in the rTOF population.3 This heterogenous scar area was likely related to the prior RV infundibular muscle resection, surgical PVR 10 years prior, and valve-in-valve TPVR struts just 4 months prior that seemed to play a part in triggering their ventricular arrhythmias.
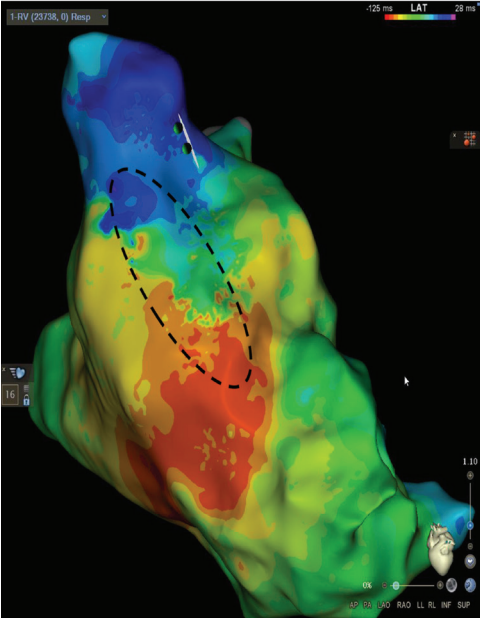
Isochronal Late Activation Mapping
The same RV pacing substrate map can be evaluated using isochronal late activation mapping (ILAM). This method identifies slowly conducting areas termed deceleration zones, which are defined as 3 or more isochrones within a 1-cm area of myocardium. In this case, a deceleration zone is located within the same SCAI seen in Figure 1 highlighted by the dashed circle in Figure 2. The use of ILAM in repaired TOF is not as well established as SCAI identification, although one recent study demonstrated a 90% correlation between identified SCAIs and ILAM deceleration zones in repaired TOF.4,5 One simple way to recognize both a SCAI and an ILAM deceleration zone is to evaluate the 1-cm area for ILAM through the anatomic isthmus identified by voltage mapping. Each isochrone can then be set to 10 ms, so if there are 3 or more isochrones through the isthmus establishing it as a deceleration zone, it can be immediately determined that the SCAI is <0.5 m/s (ie, 1 cm/30 ms = 0.33 m/s).
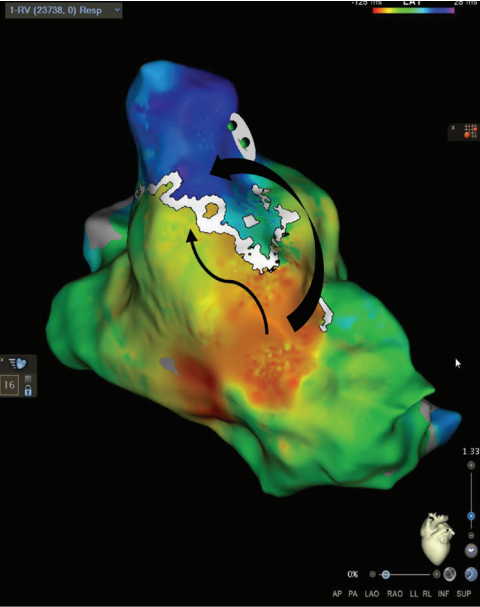
Wavefront Discontinuity Lines
The RV pacing map set was again used in a local activation time format to evaluate wavefront discontinuity lines (WADL). This recently described method of functional mapping developed by Maher et al6 uses the mapping system to automatically identify areas of slow activation and fixed lines of block to target for ablation. To visualize the lines of discontinuity, we titrated the early-meets-late setting between 25% to 35% of the mapped cycle length in the ventricle. This is visualized in Figure 3 by a white line in the same area of RVOT with activation meeting a line of block within the surgical scar emphasized by the black arrows. The WADL in this map again correlated with the same area marked as a SCAI and ILAM deceleration zone. To the author’s knowledge, there are no prior published reports of using WADL mapping in repaired TOF.
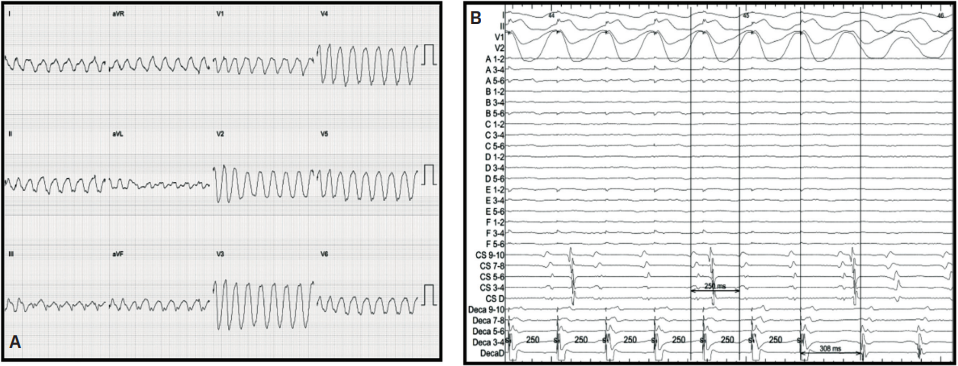
Activation and Entrainment
After examining the baseline voltage to identify surgical scar, SCAIs and functional substrate maps, a pacing catheter was then placed in the predicted VT isthmus (yellow circle, Figure 1). VT was easily induced with 2 extrastimuli off of isoproterenol. A single monomorphic tachycardia at a cycle length of 270 ms was induced originating from the RV (Figure 4A). Entrainment was performed from the catheter that was preemptively put into the isthmus at a cycle length of 250 ms, which successfully entrained the tachycardia. The post-pacing interval - tachycardia cycle length from this location was 38 ms with a small degree of manifest fusion and a stim to QRS that was extremely short (Figure 4B). We believe these findings were all consistent with the area of entrainment being in very close proximity to the exit of the tachycardia. An attempt at VT activation mapping was performed with the Optrell (Figure 5); however, this tachycardia was extremely poorly tolerated. Ablation was performed across this isthmus and within the scar followed by high-output pacing to confirm noncapture of ablated tissue. A ventricular stimulation protocol was performed with up to 3 extrastimuli from 2 locations in the RV on and off isoproterenol with noninducibility of ventricular tachyarrhythmias. At 7-month follow-up, the patient had no further VT on the same dose of dofetilide that he was previously on for atrial tachyarrhythmias.
Discussion
Tetralogy of Fallot and its variants remain the most common cyanotic congenital heart disease, and as these patients age into adulthood, the rate of ventricular arrhythmias can be as much as 15%.7 The care of this complex patient population necessitates knowledge of their hemodynamic, surgical, and arrhythmic histories to plan for the most effective electrophysiologic intervention. One special circumstance when preemptive mapping and ablation may occur for these patients is when a TPVR approach is planned. It is reasonable to perform a ventricular stimulation protocol, map for SCAIs, and ablate induced VT prior to the valve replacement, as the struts of the valve can serve as a barrier to the most arrhythmogenic substrate in rTOF.8-10 In this patient, significant right heart failure prior to TPVR prevented preemptive intervention. Interestingly, this case demonstrates that successful ablation can still be performed even after TPVR is performed.
A multifaceted approach to understanding the complicated substrate of rTOF can help guide ablation. The use of functional substrate maps is highlighted in conjunction with the standard approach of voltage identification of surgical scars and SCAIs to predict the VT circuit. The practice of functional mapping (ie, ILAM and WADL) is not well defined in this population; however, in our tertiary care center’s experience, it is useful in predicting critical components of the VT circuit in adult patients with repaired congenital heart disease. The value in this approach comes from both the low VT recurrence and minimizing time in VT during the procedure to avoid hemodynamic compromise afterward.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Drs Amoateng, Bhan, and Zimmerman have report no conflicts of interest regarding the content herein. Dr Russell reports consulting fees from Johnson & Johnson MedTech, Biotronik, and Medtronic, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Biotronik. Dr Johnson reports consulting fees from Biotronik, Medtronic, and Johnson & Johnson MedTech, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Biotronik, and support for attending meetings and/or travel from Medtronic.
References
1. Kapel GFL, Reichlin T, Wijnmaalen AP, et al. Re-entry using anatomically determined isthmuses: a curable ventricular tachycardia in repaired congenital heart disease. Circ Arrhythm Electrophysiol. 2015;8(1):102-109. doi:10.1161/CIRCEP.114.001929
2. Kapel GFL, Sacher F, Dekkers OM, et al. Arrhythmogenic anatomical isthmuses identified by electroanatomical mapping are the substrate for ventricular tachycardia in repaired Tetralogy of Fallot. Eur Heart J. 2017;38(4):268-276. doi:10.1093/eurheartj/ehw202
3. Johnson BV, Sonderman M, Magoon MJ, et al. Slowly conducting anatomic isthmuses of tetralogy of Fallot: an opportunity for ‘prophylactic’ ventricular tachycardia ablation. Heart Rhythm. 2024:S1547-5271(24)03539-2. doi:10.1016/j.hrthm.2024.10.073
4. Irie T, Yu R, Bradfield JS, et al. Relationship between sinus rhythm late activation zones and critical sites for scar-related ventricular tachycardia: systematic analysis of isochronal late activation mapping. Circ Arrhythm Electrophysiol. 2015;8(2):390-399. doi:10.1161/CIRCEP.114.002637
5. Arana-Rueda E, Acosta J, Frutos-López M, et al. Automated isochronal late activation mapping for substrate characterization in patients with repaired Tetralogy of Fallot. Europace. 2024;26(3):euae062. doi:10.1093/europace/euae062
6. Maher TR, Freedman BL, Yang S, et al. Targeting wavefront discontinuity lines for scar-related ventricular tachycardia ablation. JACC Clin Electrophysiol. 2024 Jul;10(7 Pt 1):1255-1270. doi:10.1016/j.jacep.2024.03.023
7. Khairy P, Aboulhosn J, Gurvitz MZ, et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010;122(9):868-875. doi:10.1161/CIRCULATIONAHA.109.928481
8. Nevvazhay T, Zeppenfeld K, Brouwer C, Hazekamp M. Intraoperative cryoablation in late pulmonary valve replacement for tetralogy of Fallot. Interact Cardiovasc Thorac Surg. 2020;30(5):780-782. doi:10.1093/icvts/ivaa013
9. Sabate Rotes A, Connolly HM, Warnes CA, et al. Ventricular arrhythmia risk stratification in patients with tetralogy of Fallot at the time of pulmonary valve replacement. Circ Arrhythm Electrophysiol. 2015;8(1):110-116. doi:10.1161/CIRCEP.114.001975
10. Therrien J, Siu SC, Harris L, et al. Impact of pulmonary valve replacement on arrhythmia propensity late after repair of tetralogy of Fallot. Circulation. 2001;103(20):2489-2494. doi:10.1161/01.CIR.103.20.2489











