Review Data on Lactation and ADHD Therapy
DATA UPDATE
ATTENTION: LABEL UPDATE—
Including revisions to Section 8.2 of the Qelbree (viloxazine extended-release capsules) full Prescribing Information.1
Use of ADHD treatment in lactating patients—learn more.
INDICATION
Qelbree is indicated for the treatment of ADHD in adults and pediatric patients 6 years and older.

Please see full Important Safety Information to the top left.
New mothers with ADHD face distinct challenges post pregnancy—many of which are still not fully understood or appreciated2
All new birth mothers face the challenge of hormonal changes. For a new mom with ADHD, hormonal changes can also cause ADHD symptoms to spike.3
Research on the use of ADHD medications in breastfeeding is scarce and largely limited to case reports.2
In 2019, the FDA requested that companies conduct additional research to provide clinical guidance on the level of medication transferred into breastmilk. Additionally, FDA encourages companies to examine the effect of the medication on milk production—as well as any of the risks posed by the medication on the breastfed baby.4
These clinical guidelines are important in that they are meant to help nursing mothers and their healthcare providers assess the benefits and risks of taking medication during this time when both mother and baby are adjusting to overwhelming new routines and daily demands.4
An expert review published by ACOG highlights considerations and recommendations for lactating women post pregnancy2
The article points out that clinicians should be sensitive to patient language preferences. Not all lactating women are comfortable with the term “breastfeeding,” and healthcare providers should ask people what term they prefer: breastfeeding, nursing, or chest feeding.2
The decision to breastfeed while taking medication should be made collaboratively with the patient. For women with ADHD whose treatment plan might include medication while breastfeeding, this shared decision-making process should2:
- Include a discussion about the importance of balancing the risks of using ADHD medications during breastfeeding against the potential risk for ADHD symptoms worsening if a mother is not using ADHD medication
- Ensure that the medication regimen for ADHD is appropriately adjusted and involves the minimum number of medications
- Ensure that the baby is monitored for adverse events if ADHD medication is taken during breastfeeding
Abbreviations: ACOG, American College of Obstetricians and Gynecologists; ADHD, attention-deficit/hyperactivity disorder; FDA, US Food and Drug Administration.
Qelbree is the first ADHD treatment to meet its postmarketing requirement following the 2019 FDA guidance on clinical lactation studies1

In a study of 15 healthy
lactating women receiving
Qelbree 600 mg for 3 days1:
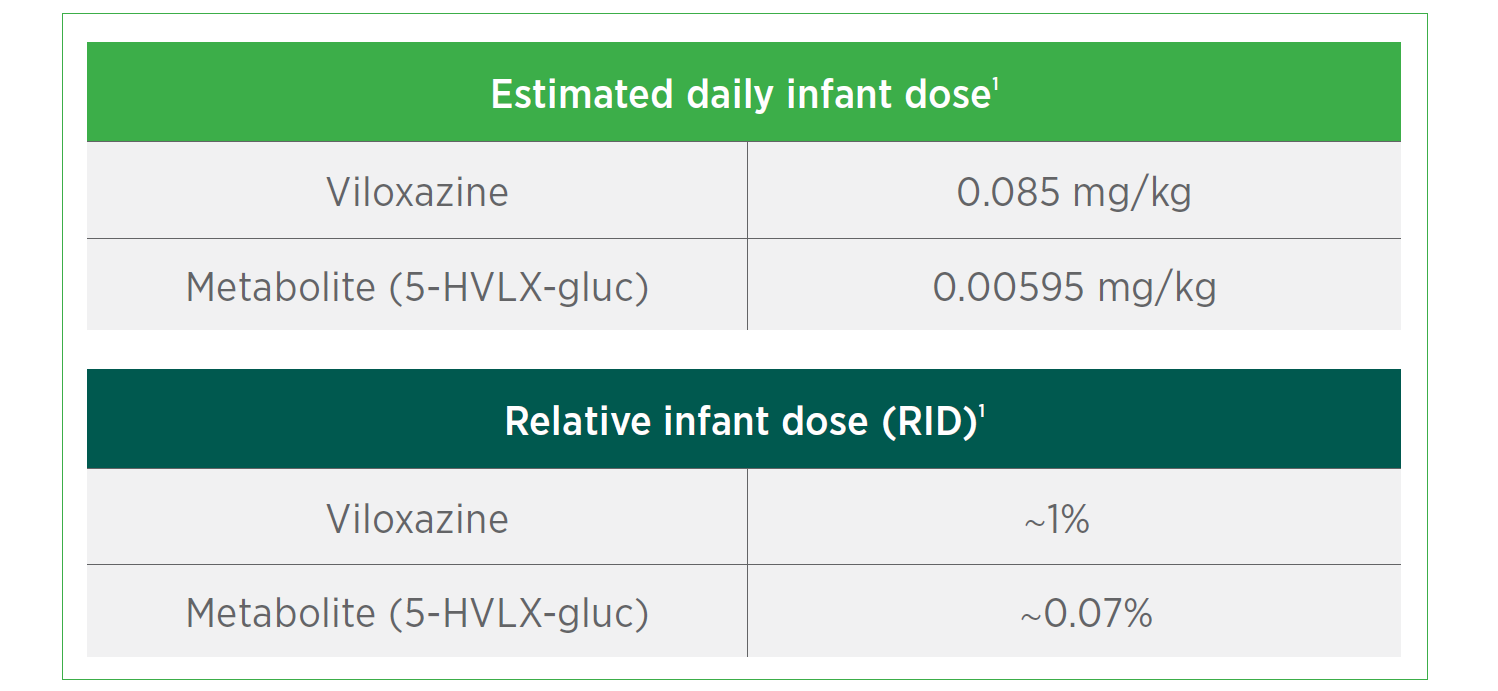
Transfer of Qelbree in breastmilk is low1*
* This study did not specifically evaluate the effects of viloxazine on breastfed infants or milk production, nor are there additional data regarding these effects. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Qelbree and any potential adverse effects on the breastfed child from Qelbree or from the underlying maternal condition.
CONTRAINDICATIONS
- Concomitant administration of a monoamine oxidase inhibitor (MAOI), or dosing within 14 days after discontinuing an MAOI, because of an increased risk of hypertensive crisis
- Concomitant administration of sensitive CYP1A2 substrates or CYP1A2 substrates with a narrow therapeutic range
Please see full Important Safety Information to the top left.
Please see full Prescribing Information, including Boxed Warning.
Qelbree (viloxazine extended-release capsules) is available in 100 mg, 150 mg, and 200 mg capsule strengths. Learn more about Qelbree, an extended-release, nonstimulant medication for ADHD: https://www.QelbreeHCP.com/
References:
- Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals, Inc.
- Scoten O, Tabi K, Paquette V, et al. Attention-deficit/hyperactivity disorder in pregnancy and the postpartum period. Am J Obstet & Gynecol. 2024;231(1):19-35.
- Wasserstein J. Menopause, Hormones & ADHD: What We Know, What Research is Needed. ADDitude ADHD Science and Strategies. Updated September 25, 2024. Accessed May 23, 2025. https://www.additudemag.com/menopause-hormones-adhd-women-research
- US Food and Drug Administration, Center for Drug Evaluation and Research. Clinical Lactation Studies: Considerations for Study Design (Draft Guidance Document). May 2019. Accessed March 20, 2025. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-lactation-studies-considerations-study-design.
QBE.2025-0028











