Dosing Optimization for a Nonstimulant ADHD Treatment – Pediatric
DATA UPDATE
Qelbree pediatric dose optimization—
SHORT- AND LONG-TERM CLINICAL OBSERVATIONS
Resource to help optimize treatment with a nonstimulant for your pediatric patients 6 years and older.
INDICATION
Qelbree is indicated for the treatment of ADHD in adults and pediatric patients 6 years and older.

Please see full Important Safety Information to the top left.
What prompts you to further optimize ADHD treatment?
Based on the phase III and OLE trials of ADHD patients 6 to 17 years of age1,2...
Is further optimization warranted for your children and teen patients with ADHD taking Qelbree?
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; OLE, open-label extension.
Pediatric phase III and OLE trial designs
Phase III methodology (P301, 302 and 303)1: Randomized, DB, placebo-controlled, fixed-dose, parallel-group, multicenter studies of children 6 to 11 years of age with ADHD (Study P301 and P303) and teens 12 to 17 years of age (Study P302), with a baseline ADHD-RS-5 Total Score ≥28, CGI-S ≥4. Study medication1: fixed dosing (100 mg/day or 200 mg/day) or matching placebo. The primary endpoint was CFB in the ADHD-RS-5 Total Score at EOS. Results1: Total scores at EOS (6 weeks) were significantly reduced with Qelbree vs placebo. The CFB in ADHD-RS-5 Total Score at EOS (Study P301) (LS mean ± SE) was -16.6 ± 1.16 for Qelbree 100 mg/day, -17.7 ± 1.12 for Qelbree 200 mg/day, and -10.9 ± 1.14 for placebo. The CFB in ADHD-RS-5 Total Score at EOS (Study P302) (LS mean ± SE) was -16.0 ± 1.45 for Qelbree 200 mg/day, -16.5 ± 1.38 for Qelbree 400 mg/day, and -11.4 ± 1.37 for placebo.
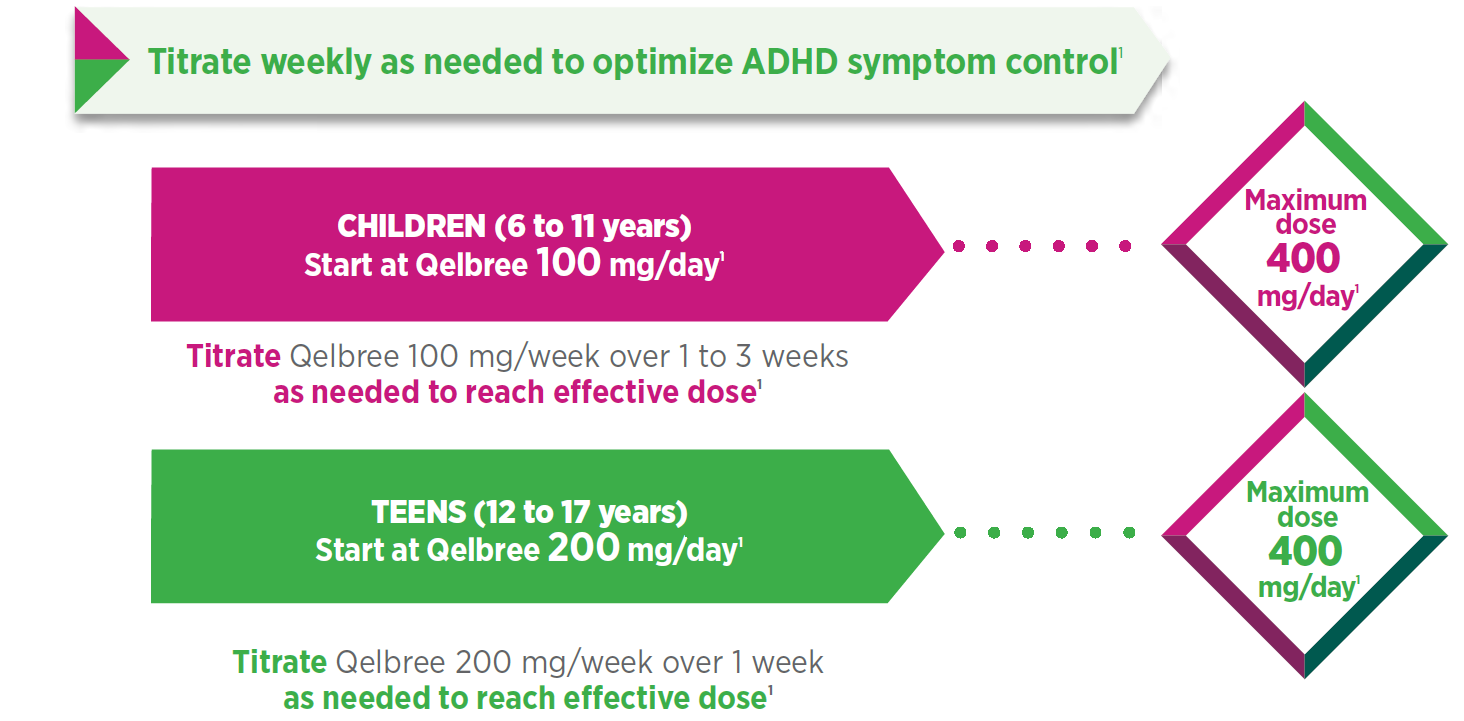
OLE methodology (P310)2: Patients who completed a previous DB study of Qelbree for the treatment of ADHD were eligible to enroll in an OLE study. Patients 6 to 11 years of age were initially treated with Qelbree 100 mg/day, and the dose could be adjusted by 100 mg/week to a range of 100 mg/day to 400 mg/day, based on clinical response.* Patients 12 to 17 years of age were initially treated with Qelbree 200 mg/day and the dose could be adjusted by 200 mg/week up to 400 mg/day.* All patients started at 100 mg/day (children) or 200 mg/day (teens) and were given 3 visits for dose optimization; visits could be 1 to 3 weeks apart, with a total optimization period of up to 12 weeks. Not all patients had all 3 optimization appointments. The interim data represent patients enrolled in the OLE as of December 31, 2020. A total sample of 708 patients received Qelbree between 100 mg and 400 mg daily, and was included in this dosing assessment. A total of 476 patients completed the 12-week dose optimization, and the corresponding patients at each monthly time point were as follows: month 3 (n=442); month 6 (n=351); month 9 (n=282); month 12 (n=245). Primary objective2: Collect long-term safety data on Qelbree monotherapy for ADHD in the pediatric population.
Interim subpopulation analysis: total daily dose of Qelbree in children and teens following 12 months of treatment in the OLE study (n=245)2†
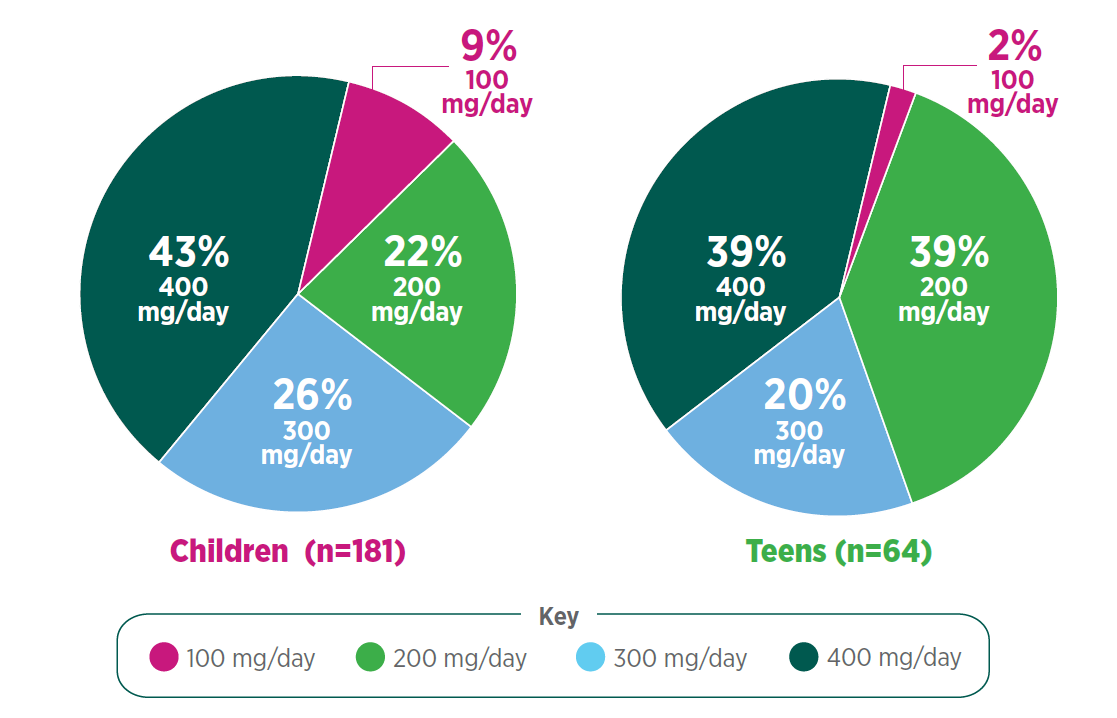
Following 12 months of treatment with once-daily Qelbree2:
- 100 mg/day was the least prevalent dose for both groups
- Nearly 30% of children and 20% of teens were taking Qelbree 300 mg/day
- Approximately 40% of children and teens were taking Qelbree 400 mg/day
> How does this compare with the pediatric patients in your practice?
*Dose will depend on response to medication. Maximum dose of Qelbree in children/teens is 400 mg/day.
† Interim data represent patients enrolled in the OLE as of December 31, 2020.
Abbreviations: ADHD-RS-5, Attention-Deficit/Hyperactivity Disorder Rating Scale, 5th Edition; CGI-S, Clinical Global Impression-Severity of Illness; DB, double blind; EOS, end of study; LS mean, least-squares mean; SE, standard error.
CONTRAINDICATIONS
- Concomitant administration of a monoamine oxidase inhibitor (MAOI), or dosing within 14 days after discontinuing an MAOI, because of an increased risk of hypertensive crisis
- Concomitant administration of sensitive CYP1A2 substrates or CYP1A2 substrates with a narrow therapeutic range
Please see full Important Safety Information to the top left.
Optimizing treatment with once-daily Qelbree is straightforward1
Once-daily Qelbree—Full-day exposure to medication in a single daily dose1,2

Administration
Capsule can be taken whole or entire contents can be sprinkled over a spoonful of soft food (pudding or applesauce). Consume the mixture in its entirety, without chewing, within 15 minutes for pudding or within 2 hours for applesauce; do not store for future use.1
- Capsules and their contents should not be cut, crushed, or chewed1
- Can be taken with or without food1
✓ Qelbree can be conveniently prescribed and refilled without a new prescription every month
✓ Qelbree has no known addiction potential or evidence of abuse1,3,4

Qelbree is available in 3 capsule strengths1
IMPORTANT SAFETY INFORMATION
- Severe renal impairment: Initiate Qelbree at 100 mg once daily and increase by 50 mg to 100 mg at weekly intervals to a maximum recommended dosage of 200 mg once daily
Please see full Important Safety Information to the top left.
Please see full Prescribing Information, including Boxed Warning.
Learn more about Qelbree, an extended-release, nonstimulant medication for ADHD: https://www.QelbreeHCP.com/
References:
- Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals, Inc.
- Data on file, Supernus Pharmaceuticals.
- Yanagita T, Wakasa Y, Kiyohara H. Drug dependence potential of viloxazine hydrochloride tested in rhesus monkeys. Pharmacol Biochem Behav. 1980;12:155-161.
- Food and Drug Administration. Table of Prescription Stimulant Label Changes. May 10, 2023. Accessed July 13, 2023. https://www.fda.gov/media/168050/download
QBE.2023-0372










