A Nonstimulant Approach in Adult ADHD
PRODUCT BULLETIN
Do you have adult ADHD patients in need of a nonstimulant treatment option? Learn more about a nonstimulant therapy approved for adults.
INDICATION
Qelbree is indicated for the treatment of ADHD in adults and pediatric patients 6 years and older.

Please see full Important Safety Information to the top left.
A Nonstimulant Approach in Adult ADHD
Qelbree® (viloxazine extended-release capsules) is an FDA-approved, novel, nonstimulant, selective norepinephrine reuptake inhibitor indicated to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in adults and pediatric patients 6 years and older.1,2
In clinical studies, Qelbree demonstrated significant reductions in ADHD symptom scores across three short-term, randomized, placebo-controlled monotherapy trials in pediatric and adolescent patients 6 to 17 years of age.1 Similar results were found in an additional trial of ADHD in adults 18 to 65 years of age.1
The most common adverse events (AEs) in pediatric patients (6-17 years) included somnolence, decreased appetite, fatigue, nausea, vomiting, insomnia, and irritability.1 In the adult trial, the most common AEs included insomnia, headache, somnolence, fatigue, nausea, decreased appetite, dry mouth, and constipation.1 Qelbree has a Boxed Warning for suicidal thoughts and behaviors, as higher rates were reported in those taking Qelbree compared to placebo in the clinical trials.1
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
- Concomitant administration of a monoamine oxidase inhibitor (MAOI), or dosing within 14 days after discontinuing an MAOI, because of an increased risk of hypertensive crisis
- Concomitant administration of sensitive CYP1A2 substrates or CYP1A2 substrates with a narrow therapeutic range
Please see full Important Safety Information to the top left.
Mechanism of Action
Qelbree contains viloxazine, in the form of viloxazine hydrochloride, which is a selective norepinephrine reuptake inhibitor.1 The viloxazine hydrochloride in Qelbree is a water-soluble, white to off-white powder formulated as extended-release oral capsules.1 Qelbree is delivered using 2-bead Microtrol™ Technology.1,3 Each capsule includes 100 mg, 150 mg, or 200 mg of viloxazine free base, which is equivalent to 115 mg, 173 mg, or 231 mg of viloxazine hydrochloride salt, respectively.1
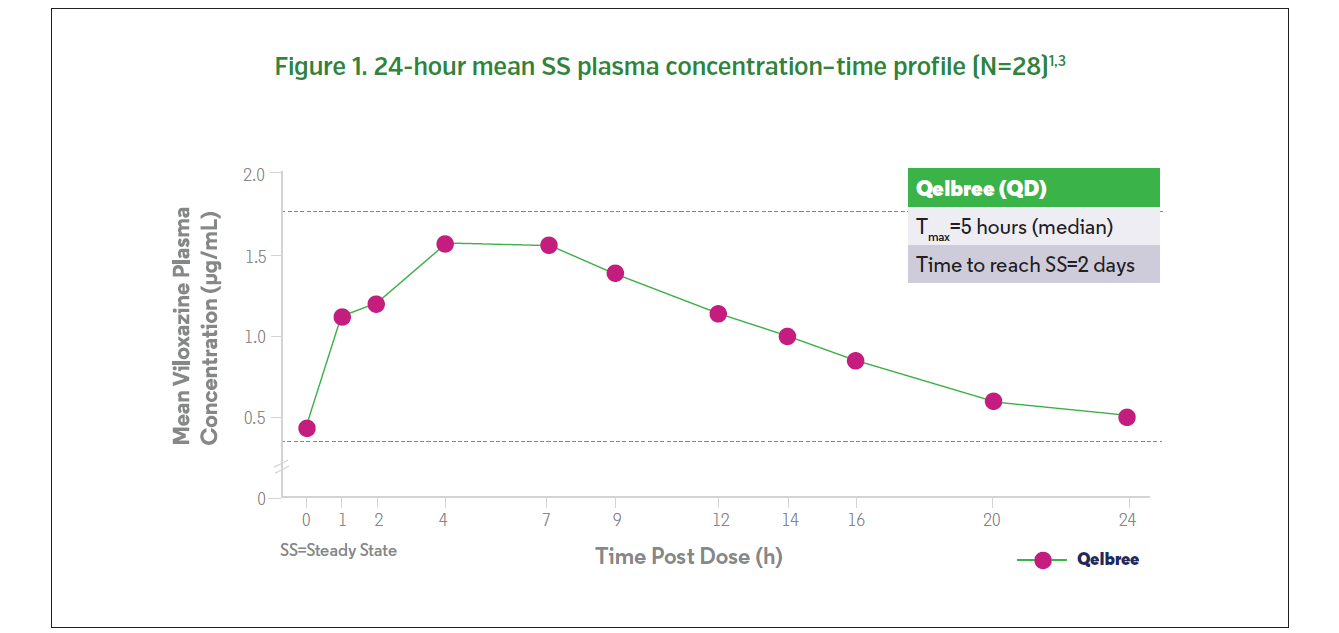
The relative bioavailability of viloxazine extended-release is about 88% compared to an immediate-release formulation.1 Following a single 200 mg dose, the median time to peak plasma concentration of viloxazine is approximately 5 (range: 3-9) hours.1 Its half-life is approximately 7 hours, and steady state is reached after two days of daily administration (Figure 1).1,3 Pre-clinical studies have indicated that viloxazine hydrochloride does not cause physical drug dependence in animal models of abuse liability.4 The potential for drug dependence of viloxazine hydrochloride was evaluated in studies in rhesus monkeys across five different conditions: single doses of viloxazine administered to healthy animals and those that were morphine- or barbital-dependent and withdrawn; repeated viloxazine doses administered to healthy animals; self-administration of viloxazine with a stimulant drug (lefetamine); and continuous administration of viloxazine.4 The results of these studies showed minimal effects of viloxazine on gross behavior, indicating a low potential of physical dependence.4 There were no reports of withdrawal symptoms or signs of dependence as AEs by patients who participated in the clinical trials.3
IMPORTANT SAFETY INFORMATION
- Suicidal thoughts and behaviors: Closely monitor all Qelbree-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy and at times of dosage changes
Please see full Important Safety Information to the top left.
Clinical Studies
The safety and efficacy of Qelbree (viloxazine extended-release capsules) was evaluated in four short-term Phase 3 trials in pediatric1,5,6, adolescent1,7, and adult patients1,8. Three randomized, double-blind, placebo-controlled, fixed-dose, parallel-group, multicenter studies were conducted in children 6 to 11 years of age with ADHD (P301: 100 and 200 mg1,5 and P303: 200 and 400 mg1,6 ) and in adolescents 12 to 17 years of age (P302: 200 and 400 mg).1,7 The fourth trial was flexible dose and was conducted in adults 18 to 65 years of age (P306: 200 to 600 mg).1,3,8
Pediatric Trial (P301)
P301 was a Phase 3, multicenter, randomized, double-blind, three-arm placebo-controlled, parallel-group, monotherapy trial in pediatric ADHD patients (6 to 11 years of age). The safety and efficacy of Qelbree treatment was evaluated across 6 weeks, including a 1-week titration and 5-week maintenance period.1 Participants were randomized to receive a once-daily, single dose of 100 mg, 200 mg, or placebo; patients were started at 100 mg during the titration period.1
The primary endpoint was the change from baseline in ADHD-RS-5 total scores at end of study (EOS).1,3 This scale includes 18 items that assess hyperactivity, impulsivity, and inattentive symptoms; higher scores indicate more severe symptoms.1,9 A key secondary endpoint was CGI-I scores at the end of the trial.5
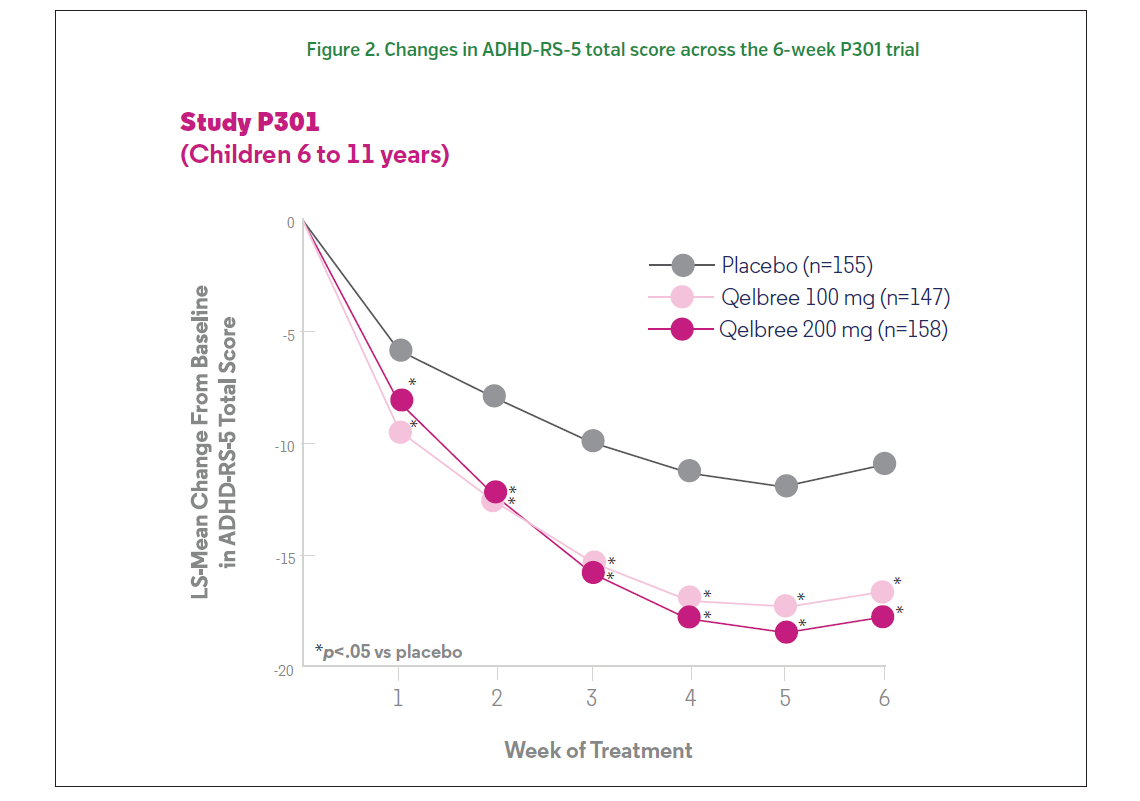
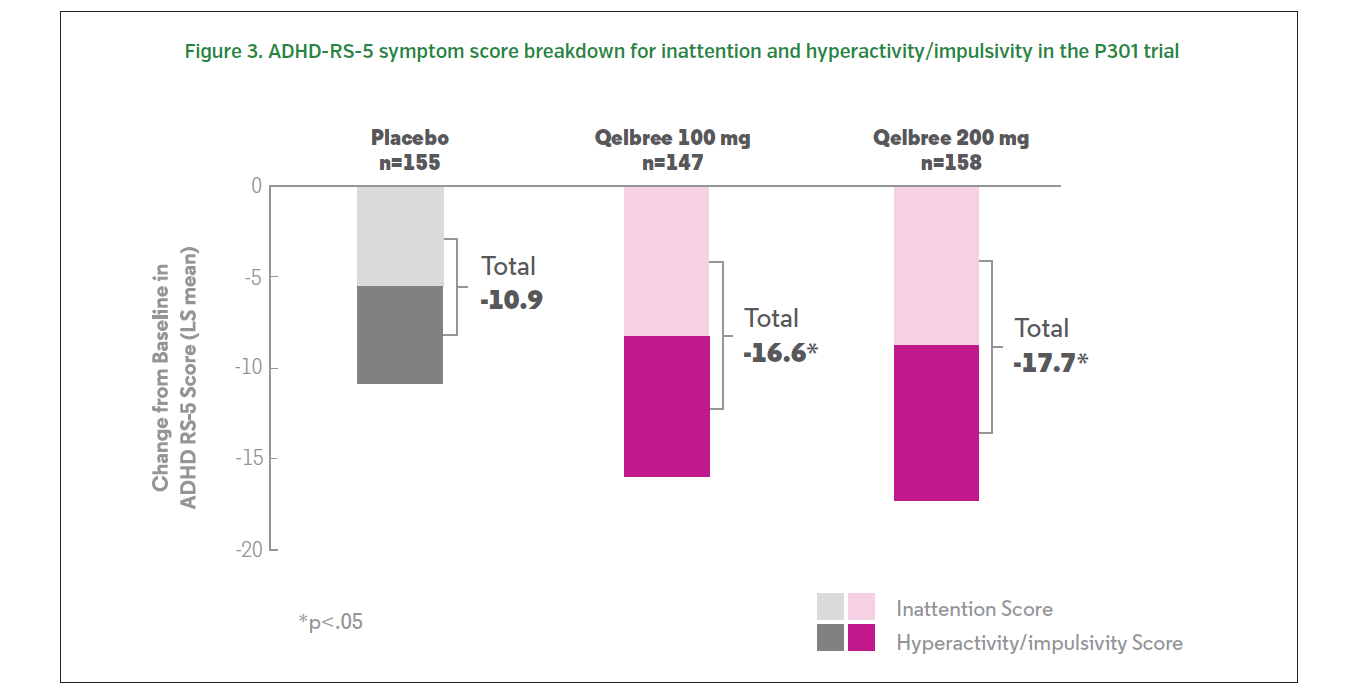
Patients treated with 100 or 200 mg of Qelbree (viloxazine extended-release capsules) experienced a statistically significantly greater reduction at the end of treatment (compared to baseline) in ADHD-RS-5 total scores compared to those who received placebo.1 The change from baseline (LS mean ± SE) in ADHD-RS-5 total score was -16.6 ± 1.16 for Qelbree 100 mg/day, -17.7 ± 1.12 for Qelbree 200 mg/day, and -10.9 ± 1.14 for placebo (Figure 2, 3).1 Total symptom score reductions were seen as early as week one during the trial.3,5 There was a statistically significantly greater reduction in CGI-I scores in those who received 100 or 200 mg of Qelbree compared to those who received placebo at the end of the study.5
IMPORTANT SAFETY INFORMATION
- Activation of mania or hypomania: Noradrenergic drugs may induce a manic or mixed episode in patients with bipolar disorder. Prior to initiating treatment with Qelbree, screen patients to determine if they are at risk for bipolar disorder. Screening should include a detailed psychiatric history, including a personal or family history of suicide, bipolar disorder, and depression
Please see full Important Safety Information to the top left.
Pediatric Trial P303
P303 was a Phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group, monotherapy trial in patients 6 to 11 years of age with ADHD. This was a confirmatory trial with a similar study design as P301 that evaluated a higher dose of Qelbree (viloxazine extended-release capsules) with a longer duration of treatment. There was a 3-week titration and a 5-week maintenance period across the 8-week trial. Participants were randomized to receive a once-daily, single dose of 200 mg, 400 mg, or placebo; patients were started at 100 mg during the titration period.1 The primary endpoint was the change from baseline in ADHD-RS-5 total scores, and a secondary endpoint was CGI-I scores at the end of treatment.1,6
The results of P303 were consistent with the data from the first pediatric trial. Patients treated with 200 or 400 mg of Qelbree experienced a statistically significantly greater reduction at the end of treatment (compared to baseline) in ADHD-RS-5 total scores compared to those who received placebo.1 The change from baseline (LS mean ± SE) in ADHD-RS-5 total score was -17.6 ± 1.43 for Qelbree 200 mg/day, -17.5 ± 1.52 for Qelbree 400 mg/day, and -11.7 ± 1.48 for placebo.1 There was a statistically significantly greater reduction in CGI-I scores in those who received 200 or 400 mg of Qelbree compared to those who received placebo at the end of treatment.1
IMPORTANT SAFETY INFORMATION
- Somnolence and fatigue: Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, due to potential somnolence (including sedation or lethargy) and fatigue, until they know how they will be affected by Qelbree
Please see full Important Safety Information to the top left.
Adolescent Trial (P302)
P302 was a Phase 3, multicenter, randomized, double-blind, three-arm, placebo-controlled, parallel-group, monotherapy trial in adolescent ADHD patients (12-17 years of age). There was a 1-week titration and 5-week maintenance period across the 6-week trial. Participants were randomized to receive a once-daily, single dose of 200 mg, 400 mg, or placebo; patients were started at 200 mg during the titration period.1 The primary endpoint was the change from baseline in total ADHD-RS-5 scores, and a secondary endpoint was CGI-I scores at the end of treatment.1,7
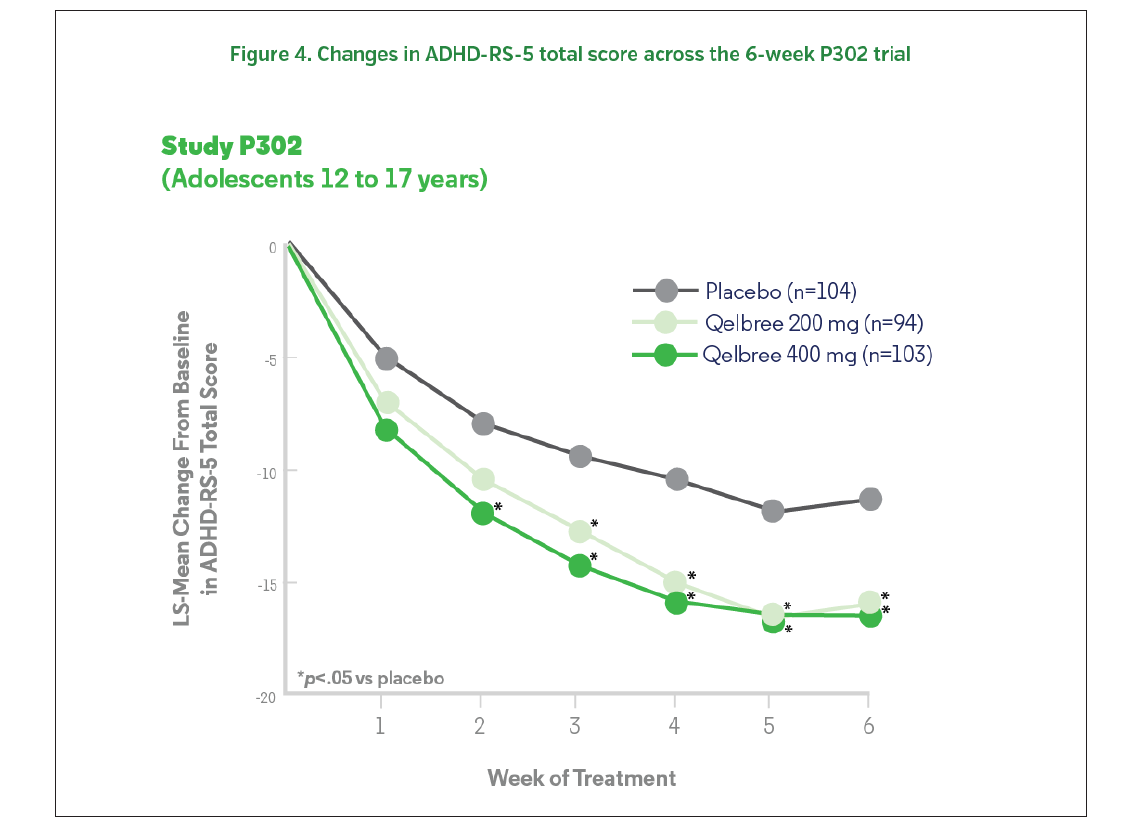
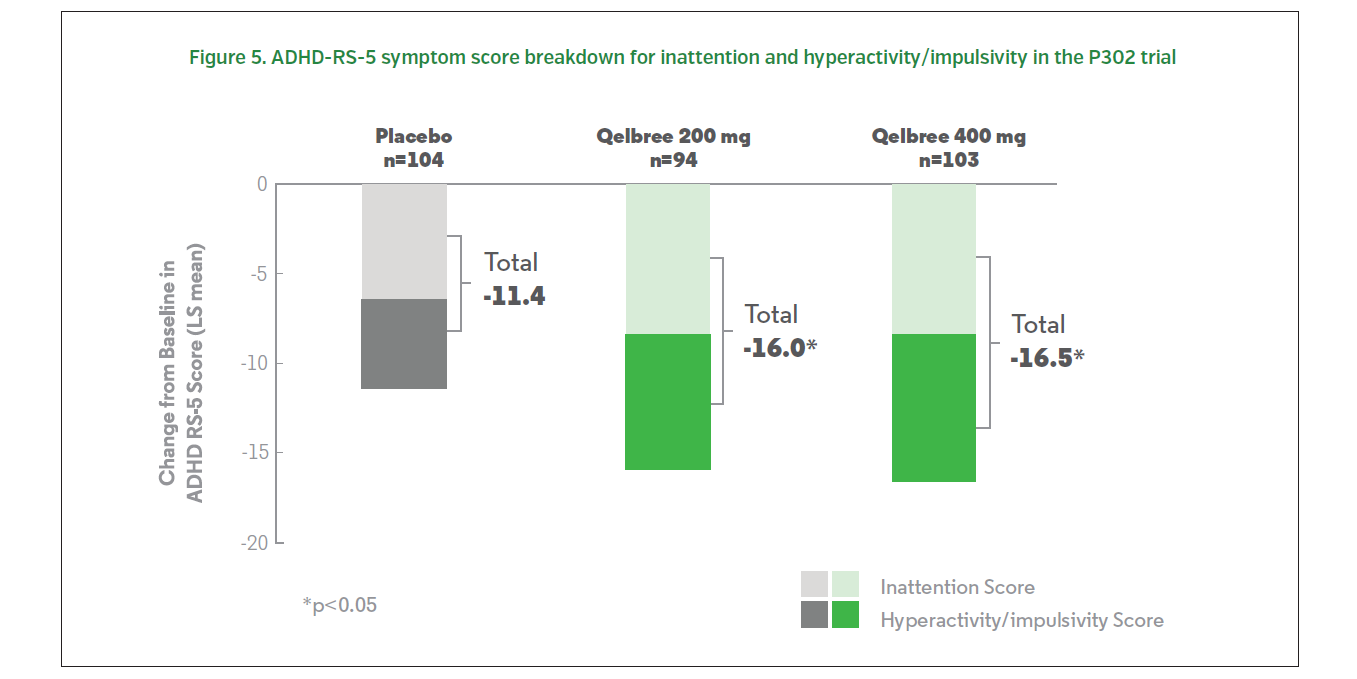
The results were similar to the pediatric trials. Patients treated with 200 or 400 mg of Qelbree experienced a statistically significantly greater reduction (compared to baseline) in ADHD-RS-5 total scores compared to those who received placebo at EOS.1 The change from baseline (LS mean ± SE) in ADHD-RS-5 total score was -16.0 ± 1.45 for Qelbree [viloxazine extended-release capsules) 200 mg/day, -16.5 ± 1.38 for Qelbree 400 mg/day, and -11.4 ± 1.37 for placebo (Figures 4, 5).1 Total symptom score reductions were seen as early as week two for the 400 mg group during the trial.7 There was a statistically significantly greater reduction in CGI-I scores for those who received 200 or 400 mg of Qelbree compared to those who received placebo at the end of the study.1
IMPORTANT SAFETY INFORMATION
- The most common adverse reactions (≥5% and at least twice the rate of placebo for any dose) in patients 6 to 17 years were somnolence, decreased appetite, fatigue, nausea, vomiting, insomnia, and irritability, and in adults, insomnia, headache, somnolence, fatigue, nausea, decreased appetite, dry mouth, and constipation.
Please see full Important Safety Information to the top left.
Adult Trial (P306)
P306 was a Phase 3, multicenter, randomized, double-blind, placebo-controlled, flexible-dose, parallel-group, monotherapy trial in adult ADHD patients (18 to 65 years of age). There was a 1-week titration and a 5-week maintenance period across the 6-week trial; patients were started at 200 mg at week one and titrated up to 400 mg at week two. The dose was adjusted 200 mg once a week (minimum dose: 200 mg; maximum dose: 600 mg). Participants were randomized to receive a once-daily, single dose between 200 mg and 600 mg or placebo.1,8
The primary endpoint was the change from baseline in the total scores of the ADHD Investigator Symptom Rating Scale (AISRS). This scale includes 18 items that assess 18 symptoms of ADHD; higher scores indicate more severe symptoms.1 The secondary endpoint was Clinical Global Impression-Severity of Illness (CGI-S) scores at the end of the trial.1
The results were consistent with both the pediatric and adolescent trials. Patients treated with Qelbree (viloxazine extended-release capsules) experienced a statistically significantly greater reduction (compared to baseline) in ADHD-RS-5 total scores compared to those who received placebo.1 While doses of Qelbree ranged from 200 mg to 600 mg across participants, the average dose at the end of the trial was 504 mg/day.1 The change from baseline (LS mean ± SE) in AISRS total scores was -15.5 ± 0.91 for Qelbree and -11.7 ± 0.90 for placebo.1 Total symptom score reductions were seen as early as week two during the trial.3 There was a statistically significantly greater reduction in CGI-S scores for those who received Qelbree compared to those who received placebo at the end of the study.1
Adverse Reactions
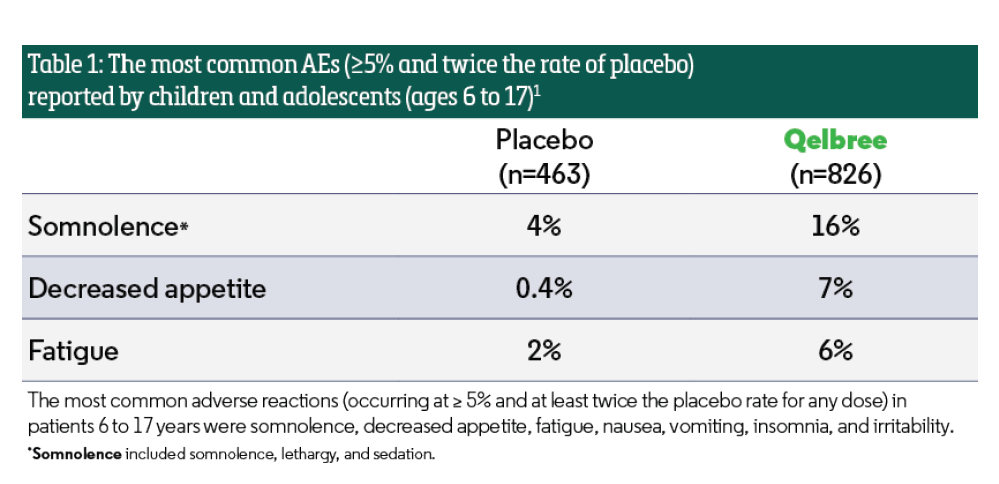
Safety was reported based on pooled data across the pediatric and adolescent trials (Qelbree: n=826; placebo: n=463). The most common AEs (≥5% and twice the rate of placebo) reported by children and adolescents ages 6 to 17 included: somnolence, decreased appetite, fatigue, nausea, vomiting, insomnia, and irritability (Table 1).1 Discontinuation rate was 3% in those who received Qelbree1 compared to 1% for placebo.3
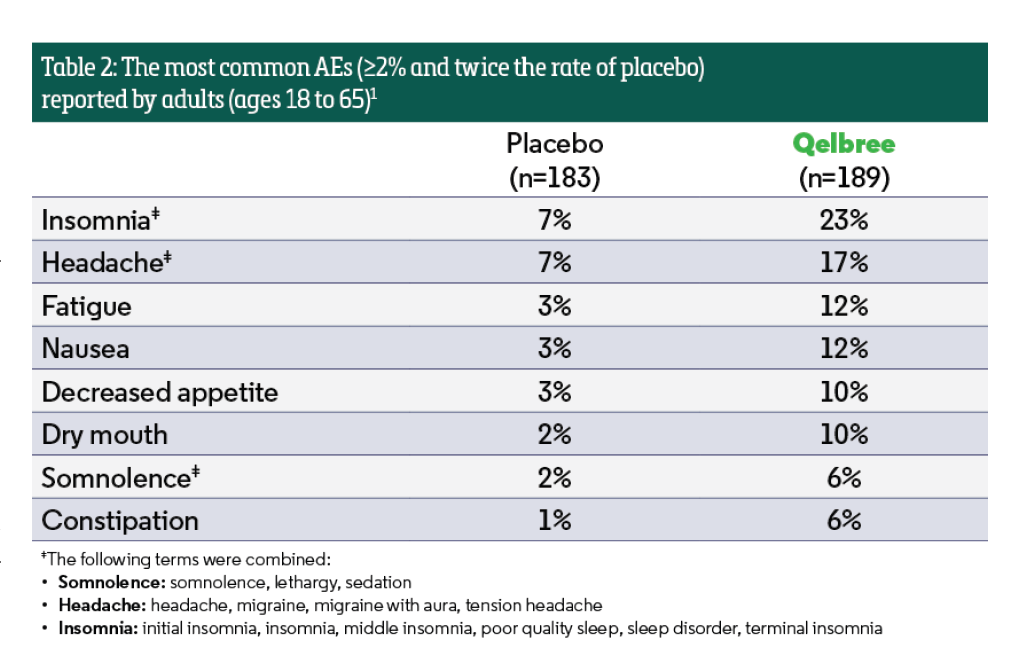
The most common AEs (≥5% and twice the rate of placebo) reported by adults ages 18 to 65 included: insomnia, headache, somnolence, fatigue, nausea, decreased appetite, dry mouth, and constipation (Table 2).1 Discontinuation was 9% in those who received Qelbree compared to 5% for placebo.3
In the clinical trials, pediatric and adult patients treated with Qelbree reported higher rates of suicidal thoughts and behaviors. Therefore, patients prescribed Qelbree must be closely monitored for clinical worsening and emergence of suicidal thoughts and behaviors.1 Heart rate and diastolic blood pressure should also be assessed prior to and during treatment, as increases in both measures were seen in the trials.1 Since medications like Qelbree (viloxazine extended-release capsules) could cause a manic or mixed episode in patients with bipolar disorder, patients should be screened for bipolar disorder risk prior to starting treatment.1 Patients should be advised not to perform activities that require mental alertness when starting treatment since Qelbree can cause somnolence and fatigue.1
IMPORTANT SAFETY INFORMATION
- Heart rate, blood pressure increases: Qelbree can cause an increase in diastolic blood pressure and heart rate. Assess these measures prior to starting therapy, following increases in dosage, and periodically during therapy
Please see full Important Safety Information to the top left.
Dosage and Administration
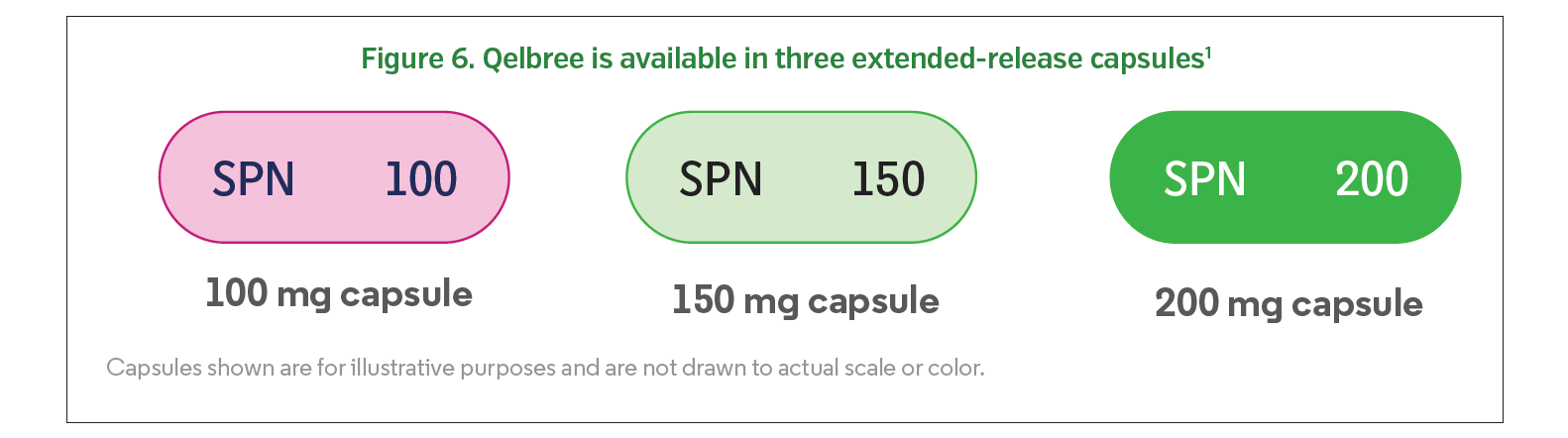
Qelbree is available in three extended-release capsules: 100 mg (yellow opaque body and cap; printed “SPN” on the cap with “100” on the body), 150 mg (lavender opaque body and cap; printed “SPN” on the cap with “150” on the body), and 200 mg (light green opaque body and cap; printed “SPN” on the cap with “200” on the body) (Figure 6).1 Capsules should be swallowed whole daily and can be taken with or without food; they should not be cut, crushed, or chewed. Alternatively, capsules can be opened, and the entire capsule contents can be sprinkled over a spoonful of pudding or applesauce. The mixture should be consumed in its entirety without chewing within 15 minutes for pudding or within 2 hours for applesauce; the mixture cannot be stored for future use.1

Please see full Important Safety Information to the top left.
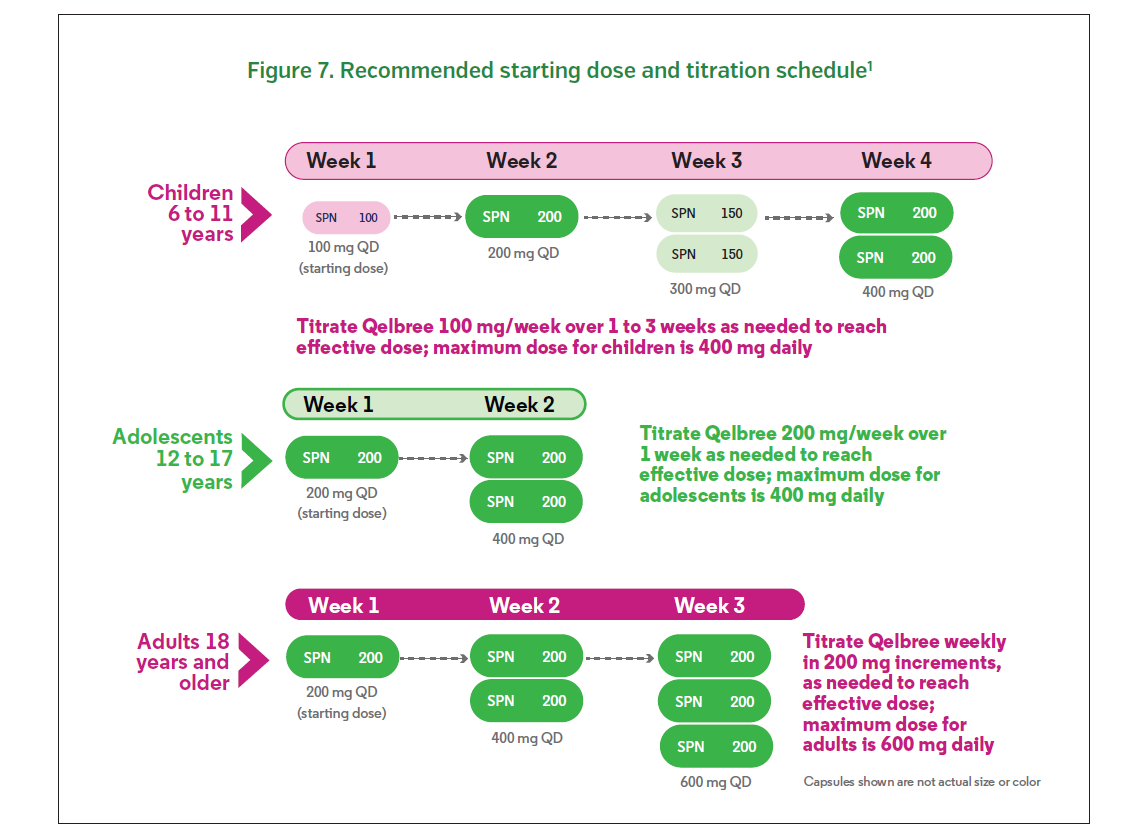
For pediatric patients 6 to 11 years of age, the recommended starting dose is 100 mg once daily (Figure 7). One week after initiating treatment, the dose can be titrated up by 100 mg increments per week to a maximum dose of 400 mg once daily, based on treatment response and tolerability.1 For adolescent patients 12 to 17 years of age, the recommended starting dose is 200 mg once daily.1 One week after initiating treatment, the dose can be titrated up by a 200 mg increment to a maximum dose of 400 mg once daily, based on treatment response and tolerability.1 For adult patients, the recommended starting dose is 200 mg once daily. One week after initiating treatment, the dose can be titrated up by 200 mg increments per week to a maximum dose of 600 mg once daily, based on treatment response and tolerability.1 Patients with severe renal impairment (EGFR < 30 mL/min/1.73m2) should start at a dose of 100 mg once daily. The dose can be titrated in weekly increments of 50 to 100 mg once daily with a maximum dose of 200 mg once daily.1
IMPORTANT SAFETY INFORMATION
- Severe renal impairment: Initiate Qelbree at 100 mg once daily and increase by 50 mg to 100 mg at weekly intervals to a maximum
recommended dosage of 200 mg once daily
Please see full Important Safety Information to the top left.
Qelbree (viloxazine extended-release capsules) is contraindicated in patients who are also taking monoamine oxidase inhibitors (MAOI), or within 14 days after discontinuing an MAOI, due to an increased risk of hypertensive crisis.1 Its use is also contraindicated in patients taking sensitive CYP1A2 substrates or CYP1A2 substrates with a narrow therapeutic range; these patients should not take Qelbree to avoid drug-drug interactions.1
Summary
Qelbree is a novel, nonstimulant medication indicated to treat ADHD in adults and pediatric patients 6 years and older.1 Qelbree contains viloxazine, in the form of viloxazine hydrochloride, which is a selective norepinephrine reuptake inhibitor.1 The viloxazine hydrochloride in Qelbree is formulated as extended-release oral capsules and is delivered using 2-bead Microtrol™ Technology.1,3
The safety and efficacy of Qelbree was evaluated in four short-term Phase 3 trials in pediatric,1,5,6 adolescent,1,7 and adult patients.1,8 Three randomized, double-blind, placebo-controlled, fixed-dose, parallel-group, multicenter studies were conducted in children 6 to 11 years of age with ADHD1,5,6 and in adolescents 12 to 17 years of age.1,7 The fourth trial was conducted in adults 18 to 65 years of age.1,8
Patients taking Qelbree demonstrated statistically significant reductions in ADHD symptoms compared to placebo across the four trials. Qelbree has a demonstrated safety and tolerability profile.1
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
- Concomitant administration of a monoamine oxidase inhibitor (MAOI), or dosing within 14 days after discontinuing an MAOI, because of an increased risk of hypertensive crisis
- Concomitant administration of sensitive CYP1A2 substrates or CYP1A2 substrates with a narrow therapeutic range
Please see full Important Safety Information to the top left.
Learn more about Qelbree, an extended-release, nonstimulant medication for ADHD: https://www.QelbreeHCP.com/
References:
- Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals, Inc.
- Food and Drug Administration. Novel drug approvals for 2021. May 13, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021.
- Data on file, Supernus Pharmaceuticals.
- Yanagita T, Wakasa Y, Kiyohara H. Drug dependence potential of viloxazine hydrochloride tested in rhesus monkeys. Pharmacol Biochem Behav. 1980;12:155-161.
- Nasser A, Liranso T, Adewole T. A phase III, randomized, placebo-controlled trial to assess the efficacy and safety of once-daily SPN-812 (viloxazine extended-release) in the treatment of attention-deficit/ hyperactivity disorder in school-age children. Clin Ther. 2020;42(8):1452-1466.
- Nasser A, Liranso T, Adewole T, et al. Once-daily SPN-812 200 and 400 mg in the treatment of ADHD in school-aged children: a phase III, randomized, controlled trial. Clin Ther. 2021;43(4)684-700.
- Nasser A, Liranso T, Adewole T. A phase III, placebo-controlled trial of once-daily viloxazine extended-release capsules in adolescents with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2021;41(4):370-380.
- Nasser A, Hull JT, Chaturvedi SA, et al. A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial Assessing the Efficacy and Safety of Viloxazine Extended-Release Capsules in Adults with Attention-Deficit/Hyperactivity Disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
- Nasser A, Kosheleff AR, Hull JT, et al. Translating Attention-Deficit/Hyperactivity Disorder Rating Scale-5 and Weiss Functional Impairment Rating Scale-Parent Effectiveness Scores into Clinical Global Impressions Clinical Significance Levels in Four Randomized Clinical Trials of SPN-812 (Viloxazine Extended-Release) in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. J Child Adolesc Psychopharmacol. 2021;31(3):214-226. doi:10.1089/cap.2020.0148
QBE.2022-0194











