Replacing Bacitracin With Vancomycin in Adams Triple Antibiotic Solution for Irrigation in Breast Implant Reconstruction: A Retrospective Review of 277 Reconstructions
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Objectives. Breast reconstruction following mastectomy commonly involves tissue expanders to create a suitable pocket for implantation. The use of triple antibiotic irrigation during tissue expander placement aims to reduce implant-related infections. Implant infections can lead to severe complications, often necessitating revision surgeries that may delay treatment of other conditions, such as cancer. For years, the most common solution used in breast pocket irrigation was the Adams solution, which comprises 80 mg gentamicin, 1 g cefazolin, and 50 000 U bacitracin in 500 mL of normal saline. However, the discontinuation of bacitracin has prompted the search for alternative formulations. Vancomycin, like bacitracin, has been shown to provide gram-positive coverage—only better. This study aims to evaluate the utility of a triple antibiotic solution (TAS) containing gentamicin, cefazolin, and vancomycin as a replacement for bacitracin in reducing infection rates following tissue expander placement.
Methods. A single-center retrospective chart review was conducted, including 152 patients who underwent a total of 277 breast reconstructions with a TAS comprising 80 mg gentamicin, 1 g cefazolin, and 1 g vancomycin from March 2020 to June 2024. Demographic and clinical data, perioperative characteristics, and postoperative outcomes—including infection rates and culture results—were analyzed.
Results. Infections occurred in 6 of 277 reconstructions (2.17% of implants). Methicillin-resistant Staphylococcus aureus was a significant pathogen in infections (50.00%). None of the systemic side effects of vancomycin, including nephrotoxicity, ototoxicity, or vancomycin flushing syndrome, were encountered. Furthermore, no evidence of drug-resistant bacteria was reported in any of the cases.
Conclusions. This study highlights the potential utility of vancomycin as an acceptable alternative to bacitracin in triple antibiotic irrigation solution following tissue expander placement post-mastectomy. Additional research could help to further establish vancomycin's efficacy and safety. If validated, this approach could be a temporary alternative to the Adams solution until a better long-term strategy is identified.
Introduction
Implant-based reconstruction continues to be the most common breast reconstruction strategy in the United States, accounting for nearly 65% of reconstructions.1 Immediate breast reconstruction with implants involves a significant risk of infection, with some studies reporting infection rates as high as 29%.2,3 The risk of infection in immediate breast reconstruction with implants is higher than in elective cosmetic breast augmentation.4 Elective cosmetic breast augmentation is a faster procedure that does not involve a primary surgeon performing a lengthy mastectomy during which breast tissue is retracted, potentially spilling bacteria endogenous to the ductal system.5,
Infection following breast reconstruction with implants can have devastating consequences. Multiple revision surgeries may be necessary: one to remove the infected tissue expander and another to replace it after the infection clears. Revision surgeries following immediate reconstruction may delay adjuvant treatment therapies.7 These operations can be physically taxing and emotionally draining, potentially adding significant financial strain, negatively impacting intimate relationships, and delaying the restoration of one’s body image.
Researchers have explored various approaches to reduce infection risk in breast reconstruction. One such approach involves the use of antibiotic irrigation during tissue expander placement. In 2001, Adams et al proposed a triple antibiotic solution (TAS) for pocket irrigation to minimize implant-related infections with a focus on decreasing the rate of capsular contracture.8 The Adams TAS consisted of 50 000 U bacitracin, 1 g cefazolin, 80 mg gentamicin, and 500 mL of normal saline, which became a preferred method for minimizing bacterial load.9,10 However, the US Food and Drug Administration (FDA) called for the discontinuation of injectable bacitracin in 2020,11 and infection rates in implant-based breast reconstruction have subsequently increased.12 Alternative formulas have since been explored, but the literature has not yet reached a consensus regarding their efficacy.10,13
In this review, we aim to present an alternative TAS containing gentamicin, cefazolin, and vancomycin. The inclusion of vancomycin was justified as a suitable alternative for bacitracin because of its activity against gram-positive bacteria and resistant strains such as methicillin-resistant Staphylococcus (S.) aureus (MRSA).14 In this review, we report our low infection rate in 277 initial-style tissue expander breast reconstructions. Additionally, we evaluate other potential complications using vancomycin instead of bacitracin.
We chose to review infection rates with vancomycin TAS in our population because immediate breast reconstruction with tissue expanders typically has an increased risk of implant infection.15 This is because it involves placing a foreign body under the mastectomy flaps,16 which are potentially less vascularized after a 2- to 4-hour surgery by another team.17 Tissue expanders may also reduce perfusion during their inflation.18 Furthermore, breast tissue is potentially contaminated by nipple-areolar complex continuity with the breast through the ductal system.5
Methods and Materials
Design and study population
After approval from the University of Louisville Institutional Review Board, we performed a retrospective chart review to identify all patients who underwent immediate breast reconstruction with tissue expanders from March 2020 to June 2024 by a single surgeon (B.J.W.) after bacitracin became unavailable. To ensure a more homogeneous cohort, all patients who underwent reconstruction using acellular dermal matrix (ADM) were excluded from the study, as the use of ADM has been linked to higher infection rates.19 Rates of tissue expander infection were analyzed, with infection being defined as infection requiring implant removal.
The alternative irrigation solution used since the discontinuation of bacitracin comprised 80 mg gentamicin, 1 g cefazolin, and 1 g vancomycin in 500 mL of normal saline. The breast pockets were irrigated for 10 minutes prior to the implantation of tissue expanders that had been submerged in solution for a minimum of 10 minutes. The 10-minute contact time was based on the US Environmental Protection Agency’s list of registered antimicrobial products effective against MRSA, most of which require a contact time of 10 minutes or less.20
Demographic and clinical data were collected, including patient age, body mass index (BMI), smoking history, comorbidities (ie, diabetes), breast pathology, and postoperative outcomes, including infection rate and culture results. Additionally, perioperative information regarding the type and number of expanders used was collected. The primary end point of this study was to present our experience using an alternative triple antibiotic irrigation solution following the FDA’s discontinuation of bacitracin.11
Statistical analysis
Descriptive statistics were used to report counts and frequencies for categorical data. Data storage and analysis were performed using Microsoft Excel (version 16.40, 2020).
Results
The patient demographics are displayed in Table 1. A total of 152 patients with ages ranging from 28 to 85 years participated in the study, with a mean age of 54.33 years (± 14.25). The mean BMI of the patients was 31.28 kg/m2 (± 8.90). Among them, 2 (1.32%) patients were underweight, 32 (21.05%) patients had a normal BMI, 44 (28.95%) patients were overweight, and 74 (48.68%) patients were classified as obese. The majority of patients had invasive ductal carcinoma (77, 50.66%), followed by ductal carcinoma in situ in 45 (29.61%) patients. Lobular carcinoma was present in 13 (8.55%) patients, while 15 (9.87%) patients were prophylactic procedures. One (0.66%) patient had papillary carcinoma, and 1 (0.66%) patient had a neuroendocrine tumor. Smoking history was reported by 31 (20.39%) patients, and 13 (8.55%) patients had a history of diabetes.
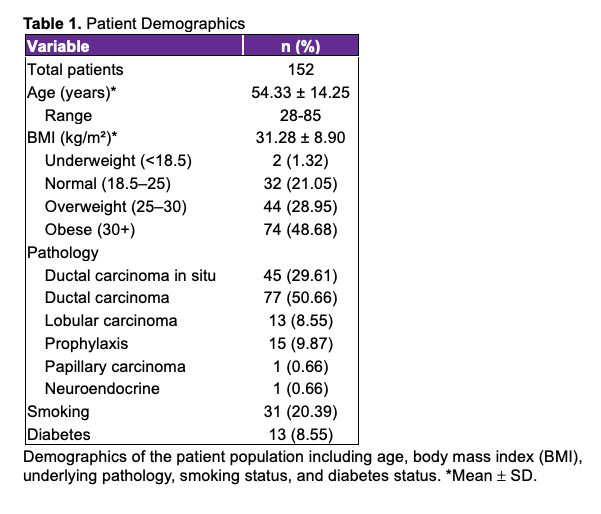
Perioperative characteristics were analyzed for 277 expanders and are presented in Table 2. Most patients (125, 82.2%) underwent bilateral mastectomy and received 2 expanders during the reconstruction, while 27 (17.8%) patients were unilateral. Textured tissue expanders were used in 116 (76.3%) patients, and smooth tissue expanders were used in 36 (23.7%) patients.
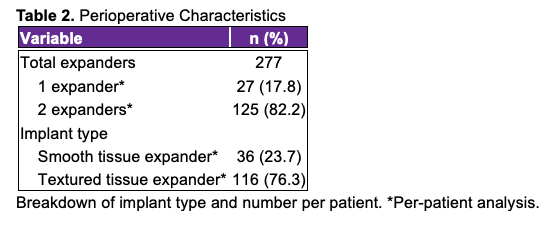
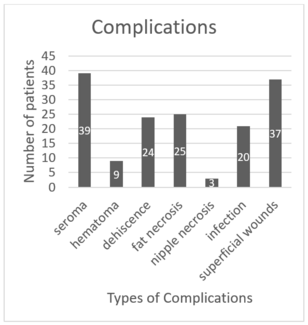
Postoperative outcomes were assessed for the 152 patients who underwent breast reconstruction and can be found in Table 3. Infections occurred in 6 (2.17%) expanders among 6 (3.95%) patients. Of these infections, 5 (1.81%) occurred following textured tissue expander implantation and 1 (0.36%) following the implantation of a smooth tissue expander. Cultures were obtained in all cases of infection; MRSA was identified as the causative agent in 3 (50.00%) cases, Methicillin-sensitive S. aureus in 2 (33.33%) cases, and skin flora in 1 (16.67%) case.
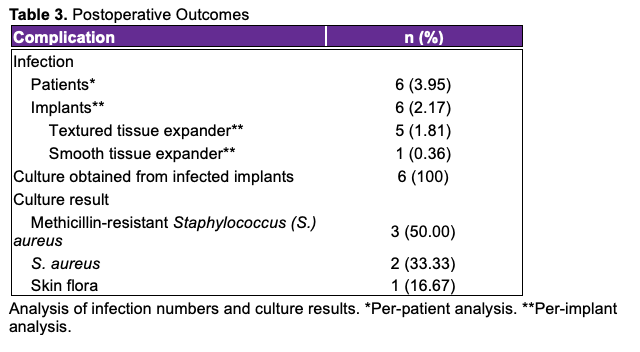
Table 4 displays the demographics for the 6 patients who suffered from perioperative infection. The data indicate that 4 (66.67%) patients were smokers and 1 (16.67%) patient was diabetic; it was noted that the diabetic patient was also a smoker. Furthermore, all 6 (100%) patients who suffered from implant infection were obese.
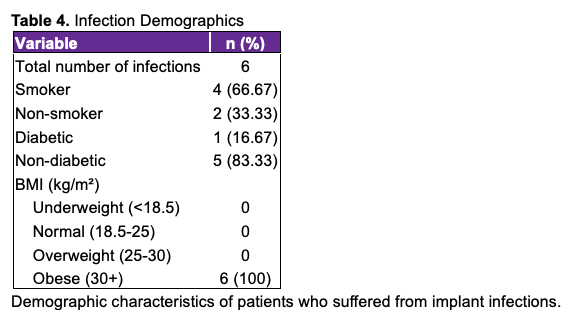
Table 5 compares infection rates with various intraoperative irrigation solutions. Rates are reported per expander, following immediate breast reconstruction with tissue expanders and without the use of ADM.9,21-23
Discussion
When bacitracin was discontinued, the Adams TAS became unavailable. This study aims to build on the Adams work and propose a suitable alternative to the original formulation.
In the Adams solution, gentamicin provided gram-negative bacterial coverage, while bacitracin covered gram-positive species.24,25 Cefazolin provides broad spectrum coverage, but the solution lost some degree of gram-positive coverage following bacitracin’s discontinuation.26,27 While bacitracin is primarily effective against gram-positive bacteria, many strains of MRSA have demonstrated resistance to bacitracin, which has been mostly attributed to the acquisition of plasmids that confer bacitracin resistance genes for the formation of altered cell wall targets or efflux pumps. Thus, bacitracin is not recommended for treatment of MRSA.28 On the other hand, vancomycin continues to be effective against MRSA through its inhibition of bacterial cell wall synthesis, providing efficacy against most strains. MRSA strains with reduced susceptibility to vancomycin have been identified in clinical settings; however, the prevalence of vancomycin-resistant S. aureus remains low.29 For these reasons, vancomycin was considered as a possible replacement for bacitracin in an alternative irrigation solution.
Vancomycin is associated with several side effects, with nephrotoxicity being chief among them. Vancomycin is almost entirely eliminated through the renal system, though the mechanism by which nephrotoxicity occurs is poorly understood.30 Nevertheless, associations between the dosage, treatment duration, and serum concentration of vancomycin and nephrotoxicity have been reported.30 In addition to nephrotoxicity, vancomycin-induced ototoxicity has emerged as a rare but noteworthy side effect of unclear etiology.31,32 Synergistic effects have been reported when vancomycin has been administered concomitantly with other potentially nephrotoxic or ototoxic drugs, such as aminoglycosides.30
Vancomycin can also cause both immediate and delayed hypersensitivity reactions. Vancomycin flushing syndrome (VFS)—historically known as red man syndrome—is the most common of these.33 In VFS, vancomycin causes the direct release of histamine from mast cells and basophils, leading to the development of an erythematous rash on the face, neck, and torso.34 While this rash is the defining characteristic of VFS, severe cases can feature angioedema, bronchospasm, and hypotension, common symptoms in type 1 hypersensitivity reactions.35 Other types of hypersensitivity reactions occur infrequently but include drug rash eosinophilia and systemic symptoms syndrome, maculopapular rash, linear IgA bullous dermatosis, and Stevens-Johnson syndrome, among others.36
It is important to note that several researchers have warned against using vancomycin in prophylactic irrigation solutions because of the possible emergence of antibiotic-resistant bacteria.8,14 Irrigation solutions may enable bacterial resistance through several mechanisms. First, antibiotics must have ample time to achieve their antibacterial functions; surgeons must therefore be diligent in allocating sufficient time for irrigation of the surgical site or risk allowing bacteria to develop resistance while exposed to solution.37-39 Second, the concentrations of antibiotics in solution must be adequately high to achieve their full effects.37 Accommodating for these 2 factors should be relatively simple if afforded proper attention. However, there is some evidence that a small amount of antibiotics from irrigation solutions can be systemically absorbed. This creates an issue where systemic bacteria may be exposed to concentrations of antibiotics that are too low to exhibit antibacterial effects, enabling resistance rather than prohibiting bacterial growth.37
Though there is controversy surrounding systemic absorption of topical antibiotics,37,39-47 the guidelines for surgical site infection (SSI) prevention provided by the Centers for Disease Control and Prevention do not contain recommendations regarding the application of local antibiotics on implants.48 Our results suggest that systemic absorption in our patients must have been low, given that none of our 152 patients experienced systemic complications of vancomycin, including—but not limited to—nephrotoxicity, ototoxicity, or VFS. Furthermore, none of our patients showed signs of bacterial resistance.
In this review, implant infection occurred in 6 of 277 (2.17%) expanders. Comorbidities were present in every patient who suffered from infection. The most frequent of these was high BMI, as all 6 patients were categorized as obese. Additionally, two-thirds (n = 4, 66.67%) of the patients with implant infections were smokers. One of these smokers was also diabetic, possessing 3 comorbidities in total. Importantly, elective plastic surgery is generally avoided in active smokers because of the increased risk of wound complications. For this reason, the senior author generally does not place tissue expanders in patients who admit to having smoked within 3 months of the procedure date. When cancer surgery and reconstruction are necessary, smoking cessation is strongly emphasized for at least 1 month prior to any intervention by the oncological team and the plastic surgery service. However, not all patients are entirely forthcoming about their smoking status, or they have relapses prior to surgery. Patients who develop wound complications are questioned about nicotine and tested to confirm use. These comorbidities are potential confounding variables when assessing the efficacy of the modified Adams solution.
Comparisons between infection rates with varying irrigation solutions can be made between the studies listed in Table 5. From each study, it is possible to deduce an infection rate (infection requiring implant removal) per implant among patients undergoing immediate, implant-based breast reconstruction with tissue expanders and without the use of ACM, though careful interpretation of the literature is required. Any study that did not clearly meet these criteria was excluded. Several factors limited our ability to include studies for comparison to our own results. First, many studies reported their infection rates in terms of patients rather than total number of breast reconstructions; this makes it challenging to compare results, given that many patients undergo bilateral reconstruction and therefore have 2 reconstructions, resulting in dramatically altered infection rates. Second, many studies did not provide a clear and consistent definition as to what constitutes an infection, making it difficult to compare the value of 1 irrigation solution over another. We chose to define infection as necessitating the removal of the tissue expander because implant removal is generally the most burdensome infection-related complication for patients and, thus, is the main outcome that we hope to avoid. It would be intellectually dishonest to compare our results, which include only major infections, to those that included minor infections (eg, cellulitis) that were corrected with a simple course of oral antibiotics. Additionally, numerous studies included patients who underwent reconstruction using ADM, which has been associated with increased infection risk.19 Even in cases where data on infection rates in each subgroup (immediate reconstruction, with tissue expanders, without ADM, etc) were provided, the information was often not presented in a manner that allowed us to determine rates for patients who fit into all necessary subgroups. For example, if a study reported infection rates for immediate reconstruction and no-ADM categories separately, it was often impossible to determine how many patients with infection were overlapping between groups simultaneously. These limitations significantly hindered our ability to include studies that could reasonably be compared to our own results. Some of the studies in Table 5 that had a high rate of infection were conducted decades ago; it is possible that their infection rates were comparatively higher given the rapid advancement in the health care field since these studies were published. Unfortunately, most recent studies lack the methodological clarity needed for inclusion because of confounding from mixed reconstructive techniques, ADM use, or definition of infection, limiting their utility for comparison. Our research helps to address these gaps in the literature, highlighting the need for uniform definitions and quality analysis of more homogenized groups in the future.
Our results indicate that a modified Adams solution containing gentamycin, cefazolin, and vancomycin may provide a suitable alternative to the original Adams TAS in patients undergoing tissue expander placement, given that our rate of infection of 2.17% is lower than most others reported in Table 5. It is important to acknowledge potential confounding variables that may influence the effect of the modified Adams solution on infection rates. Following a 3- to 4-hour mastectomy performed by another team, the sterility of the operative field at the time of reconstruction may be uncertain. To address this concern, a full re-prepping and re-draping of the surgical site is meticulously performed by the surgeon prior to each reconstruction. During this procedure, the surgeon temporarily staples the mastectomy wound closed and re-preps the skin using 2 chlorhexidine gluconate swabsticks per side, applied medially to laterally. Re-draping is performed using adhesive tapes (instead of staples), a universal drape kit, 10 x 10 drapes, and approximately 30 towels to establish a new sterile field. After the new drapes are placed, the temporary staples and internal laparotomy sponges are removed using a hemostat and surgical pickups and are discarded along with the swabsticks. At this point, the surgeon changes into new sterile gloves and initiates the 10-minute irrigation process with the modified Adams solution. Furthermore, ACM is avoided at the first stage, and the new expander is bathed in the TAS for 10 minutes. All mastectomy wound edges are excisionally freshened as well. Patients receive prophylactic intravenous antibiotics beginning immediately before surgery and continuing until the surgical drains are removed, typically at 2 weeks, provided the drain output is less than 30 mL per hour. While bacitracin irrigation solution is no longer available, it is worth noting that, to our gross recollection, our infection rates during its use were similar to those observed when using the modified Adams solution.
The 1 study included in Table 5 with an infection rate lower than ours was performed by Adams himself. However, one of his irrigation solutions contained bacitracin, which is now unavailable, and the other contained Betadine (Atlantis Consumer Healthcare Inc, previously Avrio Health LP), which is a controversial agent in irrigation solutions.
Prior to proposing his TAS, Adams recommended Betadine-based breast pocket irrigation.49 Shortly afterwards, the FDA restricted the use of Betadine because of concerns regarding implant rupture, prompting Adams to suggest his TAS.8 Nevertheless, many plastic surgeons were skeptical of the FDA’s decision and continued to use Betadine in breast pocket irrigation.50 In 2017, a communication from the FDA rescinded their ban of Betadine use with Natrelle (Allergan Aesthetics, an AbbVie Company) brand implants.51 Many physicians have interpreted this as authorization to resume the use of Betadine in irrigation solutions. It is important to note that the FDA communication merely reversed the ban of Betadine for Natrelle implants; the FDA did not approve the use of Betadine in breast pocket irrigation, and never has.52
Aside from the lack of FDA approval, we have refrained from using Betadine in this context because of underlying safety concerns; Betadine is not sterile and is marketed as a topical antiseptic for external use only.52 Both Pseudomonas aeruginosa and Burkholderia cepacia have been identified previously as intrinsic contaminants in povidone-iodine solutions, illustrating this fact.53 Additionally, Betadine has cytotoxic effects and has been shown to damage human fibroblasts and impair wound healing in animals.54 In light of these concerns, we opted for the use of a TAS.
Of the irrigation solutions proposed by Adams following the FDA’s Betadine ban, there were only 3 that were completely effective at controlling the growth of all organisms except for a small amount of Pseudomonas.8 Following the discontinuation of bacitracin, only one remains unrestricted by the FDA. This solution—comprising vancomycin, gentamicin, and cefazolin—was the one evaluated in this study. According to Adams, this solution was slightly more effective than the bacitracin-based solution.8 However, Adams ultimately recommended the bacitracin-containing solution because of the aforementioned concerns regarding vancomycin.8 When faced with a lack of alternative solutions that are similarly capable of preventing infection, the decision to abstain from using vancomycin is harder to make. This is particularly true given that gram-positive bacteria, which are generally susceptible to vancomycin, have been found to cause the majority of SSIs following placement of breast tissue expanders.55 One study, for instance, showed that gram-positive species caused 73% of SSIs in tissue expander placement.55 Given these factors, our results indicate that the use of vancomycin may be an acceptable alternative until a safer long-term strategy is identified.
Limitations
The primary limitation of this study is the small sample size and the presence of a varied sample population with numerous confounding comorbidities. These factors may have influenced the results and limited the generalizability of the findings. Therefore, caution should be exercised in interpreting the results, and further research with larger and more homogenous cohorts is needed to validate the efficacy of the TAS intraoperatively.
Conclusions
Our study suggests that vancomycin is an acceptable alternative to bacitracin in the traditional Adams TAS. In our review of 277 reconstructions, none of our patients developed complications related to vancomycin, and our infection rates were lower than most reported.
Acknowledgments
Authors: Quinton L. Carr, BA1; Colton H. Connor, BS1; Ryan Cantrell, BS1; Shriya Dodwani, BA1; Alexander L. Mostovych, MS1; Marcus Bennett, BA1; Samuel L Corey, MD2; Dexter W. Weeks, MD3; Anthony Azzolini, MD3; Carter Prewitt, MD3; Bradon J. Wilhelmi, MD3
Affiliations: 1University of Louisville School of Medicine, Louisville, Kentucky; 2Community Health Network, Indianapolis, Indiana; 3Division of Plastic and Reconstructive Surgery, Department of Surgery, University of Louisville, Louisville, Kentucky
Correspondence: Bradon J. Wilhelmi, MD, Division of Plastic and Reconstructive Surgery, Department of Surgery, University of Louisville, 550 South Jackson Street, ACB 2nd Floor, Louisville, KY 40202, USA. Email: bradon.wilhelmi@louisville.edu
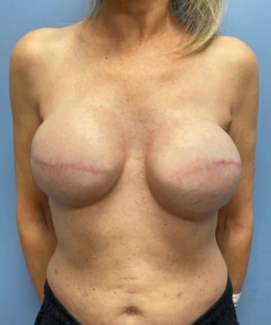
Ethics: The patients in this document have been provided informed consent on the use of their images and granted the use of their images for scientific publications. The study was approved by the University of Louisville Human Subjects Research and Institutional Review Board (IRB), IRB# 17.0522.
Disclosures: The authors disclose no relevant financial or nonfinancial interests.
References
- Bertozzi N, Pesce M, Santi P, Raposio E. Tissue expansion for breast reconstruction: methods and techniques. Ann Med Surg (Lond). 2017;21:34-44. doi:10.1016/j.amsu.2017.07.048
-
Warren Peled A, Itakura K, Foster RD, et al. Impact of chemotherapy on postoperative complications after mastectomy and immediate breast reconstruction. Arch Surg. 2010;145(9):880-885. doi:10.1001/archsurg.2010.163
-
Phillips BT, Bishawi M, Dagum AB, Khan SU, Bui DT. A systematic review of antibiotic use and infection in breast reconstruction: what is the evidence? Plast Reconstr Surg. 2013;131(1):1-13. doi:10.1097/PRS.0b013e3182729c39
-
Long C, Sue GR, Chattopadhyay A, Huis In’t Veld E, Lee GK. Critical evaluation of risk factors of infection following 2-stage implant-based breast reconstruction. Plast Reconstr Surg Glob Open. 2017;5(7):e1386. doi:10.1097/GOX.0000000000001386
-
Bartsich S, Ascherman JA, Whittier S, Yao CA, Rohde C. The breast: a clean-contaminated surgical site. Aesthet Surg J. 2011;31(7):802-806. doi:10.1177/1090820X11417428
-
Goethals A, Menon G, Rose J. Mastectomy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. Updated November 10, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559068/
-
Petit J, Rietjens M, Garusi C. Breast reconstructive techniques in cancer patients: which ones, when to apply, which immediate and long term risks? Crit Rev Oncol Hematol. 2001;38(3):231-239. doi:10.1016/S1040-8428(00)00137-2
-
Adams WP Jr, Conner WC, Barton FE Jr, Rohrich RJ. Optimizing breast-pocket irrigation: the post-betadine era. Plast Reconstr Surg. 2001;107(6):1596-1601. doi:10.1097/00006534-200105000-00049
-
Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;117(1):30-36. doi:10.1097/01.prs.0000194903.67028.29
-
Epps MT, Langsdon S, Pels TK, et al. Pocket irrigation and technique during reconstructive surgery: an American Society of Plastic Surgery survey of current practice. Ann Plast Surg. 2019;82(6S Suppl 5):S427-S432. doi:10.1097/SAP.0000000000001790
-
US Food and Drug Administration. FDA requests withdrawal of bacitracin for injection from market. January 31, 2020. Accessed December 17, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-bacitracin-injection-market
-
Oleru OO, Akhavan AA, Seyidova N, Ibelli T, Taub PJ, Henderson P. Did the national ban on bacitracin irrigation affect infection rates in implant-based breast reconstruction? An analysis of a national database. Clin Breast Cancer. 2023;23(3):e103-e108. doi:10.1016/j.clbc.2022.12.019
-
Tirrell AR, Bekeny JC, Tefera EA, Song DH, Fan KL. Bacitracin for injection recall: impact on immediate breast implant surgical outcomes. Breast J. 2022;2022:1389539. doi:10.1155/2022/1389539
-
Patel S, Preuss CV, Bernice F. Vancomycin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. Updated October 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459263/
-
von Glinski M, Holler N, Kümmel S, et al. Autologous vs implant-based breast reconstruction after skin- and nipple-sparing mastectomy—a deeper insight considering surgical and patient-reported outcomes. Front Surg. 2022;9:903734. doi:10.3389/fsurg.2022.903734
-
Jansen B, Peters G. Foreign body associated infection. J Antimicrob Chemother. 1993;32(suppl A):69-75. doi:10.1093/jac/32.suppl_a.69
-
Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132(9):997-1004. doi:10.1001/archsurg.1997.01430330063010
-
Armstrong RW, Berkowitz RL, Bolding F. Infection following breast reconstruction. Ann Plast Surg. 1989;23(4):284-288. doi:10.1097/00000637-198910000-00003
-
Chun YS, Verma K, Rosen H. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125(2):429-436. doi:10.1097/PRS.0b013e3181c82d90
-
United States Environmental Protection Agency. EPA’s registered antimicrobial products effective against methicillin-resistant Staphylococcus aureus (MRSA) and/or vancomycin-resistant Enterococcus faecalis/faecium [List H]. Updated June 16, 2025. Accessed December 21, 2024. https://www.epa.gov/pesticide-registration/epas-registered-antimicrobial-products-effective-against-methicillin
-
Schuster RH, Rotter S, Boonn W, Efron G. The use of tissue expanders in immediate breast reconstruction following mastectomy for cancer. Br J Plast Surg. 1990;43(4):413-418. doi:10.1016/0007-1226(90)90005-K
-
Chen CF, Lin SF, Hung CF, Chou P. Risk of infection is associated more with drain duration than daily drainage volume in prosthesis-based breast reconstruction: a cohort study. Medicine (Baltimore). 2016;95(49):e5605. doi:10.1097/MD.0000000000005605
-
Nahabedian MY, Tsangaris T, Momen B, Manson PN. Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg. 2003;112(2):467-476. doi:10.1097/01.PRS.0000070727.02992.54
-
Chaves BJ, Tadi P. Gentamicin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. Updated April 10, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557550/
-
Nguyen R, Khanna NR, Safadi AO, et al. Bacitracin Topical. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. Updated June 8, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536993/
-
Hunsicker LM, Chavez-Abraham V, Berry C, McEwen D. Efficacy of vancomycin-based continuous triple antibiotic irrigation in immediate, implant-based breast reconstruction. Plast Reconstr Surg Glob Open. 2017;5(12):e1624. doi:10.1097/GOX.0000000000001624
-
Bui T, Patel P, Preuss CV. Cephalosporins. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. Updated February 17, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551517/
-
Suzuki M, Yamada K, Nagao M, et al. Antimicrobial ointments and methicillin-resistant Staphylococcus aureus USA300. Emerg Infect Dis. 2011;17(10):1917-1920. doi:10.3201/eid1710.101365
-
Tawfeek CE, Khattab S, Elmaraghy N, Heiba AA, Nageeb WM. Reduced vancomycin susceptibility in Staphylococcus aureus clinical isolates: a spectrum of less investigated uncertainties. BMC Infect Dis. 2024;24(1):1218. doi:10.1186/s12879-024-10047-2
-
Zamoner W, Prado IRS, Balbi AL, Ponce D. Vancomycin dosing, monitoring and toxicity: critical review of the clinical practice. Clin Exp Pharmacol Physiol. 2019;46(4):292-301. doi:10.1111/1440-1681.13066
-
Humphrey C, Veve MP, Walker B, Shorman MA. Long-term vancomycin use had low risk of ototoxicity. PLoS One. 2019;14(11):e0224561. doi:10.1371/journal.pone.0224561
-
Rybak LP, Ramkumar V, Mukherjea D. Ototoxicity of non-aminoglycoside antibiotics. Front Neurol. 2021;12:652674. doi:10.3389/fneur.2021.652674
-
Alvarez-Arango S, Yerneni S, Tang O, et al. Vancomycin hypersensitivity reactions documented in electronic health records. J Allergy Clin Immunol Pract. 2021;9(2):906-912. doi:10.1016/j.jaip.2020.09.027
-
Martel TJ, Jamil RT, King KC. Vancomycin Flushing Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. Updated 2024. https://www.ncbi.nlm.nih.gov/books/NBK482506/
-
Zhu LJ, Liu AY, Wong PH, Arroyo AC. Road less traveled: drug hypersensitivity to fluoroquinolones, vancomycin, tetracyclines, and macrolides. Clin Rev Allergy Immunol. 2022;62(3):505-518. doi:10.1007/s12016-021-08919-5
-
Kayode OS, Rutkowski K. Vancomycin hypersensitivity: it is not always what it seems. J Allergy Clin Immunol Pract. 2021;9(2):913-915. doi:10.1016/j.jaip.2020.10.040
-
Edmiston CE Jr, Leaper D, Spencer M, et al. Considering a new domain for antimicrobial stewardship: topical antibiotics in the open surgical wound. Am J Infect Control. 2017;45(11):1259-1266. doi:10.1016/j.ajic.2017.04.012
-
Fry DE. Topical antimicrobials and the open surgical wound. Surg Infect (Larchmt). 2016;17(5):520-524. doi:10.1089/sur.2016.107
-
Barnes S, Spencer M, Graham D, Johnson HB. Surgical wound irrigation: a call for evidence-based standardization of practice. Am J Infect Control. 2014;42(5):525-529. doi:10.1016/j.ajic.2014.01.012
-
Oakley RE, Nimer KA, Bukhari E. Is the use of topical vancomycin to prevent mediastinitis after cardiac surgery justified? J Thorac Cardiovasc Surg. 2000;119(1):190-191. doi:10.1016/S0022-5223(00)70248-0
-
Li Bassi G, Saucedo LM. The use of prophylactic vancomycin to prevent MRSA colonization: does this double-edged sword promote future vancomycin resistance or is it a safe preventative strategy that should be used in all patients in the context of MRSA endemicity? Minerva Anestesiol. 2010;76(3):175-177. PMID:20125092
-
Johnson JD, Nessler JM, Horazdovsky RD, Vang S, Thomas AJ, Marston SB. Serum and wound vancomycin levels after intrawound administration in primary total joint arthroplasty. J Arthroplasty. 2017;32(3):924-928. doi:10.1016/j.arth.2015.10.015
-
Abdullah KG, Attiah MA, Olsen AS, Richardson A, Lucas TH. Reducing surgical site infections following craniotomy: examination of the use of topical vancomycin. J Neurosurg. 2015;123(6):1600-1604. doi:10.3171/2014.12.JNS142092
-
Abdullah KG, Chen HI, Lucas TH. Safety of topical vancomycin powder in neurosurgery. Surg Neurol Int. 2016;7(suppl 39):S919-S926. doi:10.4103/2152-7806.195227
-
Desmond J, Lovering A, Harle C, Djorevic T, Millner R. Topical vancomycin applied on closure of the sternotomy wound does not prevent high levels of systemic vancomycin. Eur J Cardiothorac Surg. 2003;23(5):765-770. doi:10.1016/S1010-7940(03)00033-2
-
Deora H, Nagesh M, Garg K, Singh M, Chandra SP, Kale SS. Topical vancomycin for prevention of surgical site infection in cranial surgeries: results of an updated systematic review, meta-analysis and meta-regression. Neurol India. 2023;71(5):875-883. doi:10.4103/0028-3886.388107
-
O’Toole RV, Degani Y, Carlini AR, Castillo RC, O’Hara NN, Joshi M; METRC. Systemic absorption and nephrotoxicity associated with topical vancomycin powder for fracture surgery. J Orthop Trauma. 2021;35(1):29-34. doi:10.1097/BOT.0000000000001866
-
Berríos-Torres SI, Umscheid CA, Bratzler DW, et al; Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784-791. doi:10.1001/jamasurg.2017.0904
-
Adams WP Jr, Conner WC, Barton FE Jr, Rohrich RJ. Optimizing breast pocket irrigation: an in vitro study and clinical implications. Plast Reconstr Surg. 2000;105(1):334-338. doi:10.1097/00006534-200001000-00051
-
Wiener TC. Betadine and breast implants: an update. Aesthet Surg J. 2013;33(4):615-617. doi:10.1177/1090820X13484036
-
US Food and Drug Administration. Premarket approval (PMA). US Department of Health and Human Services. Accessed August 13, 2024. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?ID=402786
-
Swanson E. It is time to abandon betadine irrigation of breast implant pockets. Ann Plast Surg. 2022;88(2):131-132. doi:10.1097/SAP.0000000000003051
-
Chang CY, Furlong LA. Microbial stowaways in topical antiseptic products. N Engl J Med. 2012;367(23):2170-2173. doi:10.1056/NEJMp1212680
-
Lineaweaver W, Howard R, Soucy D, et al. Topical antimicrobial toxicity. Arch Surg. 1985;120(3):267-270. doi:10.1001/archsurg.1985.01390270007001
-
Molska M, Wichtowski M, Murawa D. Microbiology of breast tissue expanders. Contemp Oncol (Pozn). 2021;25(4):291-294. doi:10.5114/wo.2021.112561