Prevalence of Biofilm in Chronic Wounds: Systematic Review With Meta-Analysis
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. To estimate the prevalence of biofilms in chronic wounds. Methods. The authors performed a systematic review of prevalence studies and meta-analysis, structured according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines. Articles were searched in Scopus (Elsevier), Web of Science (Clarivate), MEDLINE/PubMed (National Institutes of Health), and Embase (Elsevier) databases. Data collected included the author and year of publication, total number of lesions evaluated, number of lesions with biofilm, detected bacteria, biofilm levels, country where the research was conducted, and the methodological quality of the studies. The meta-analysis was performed using a random effects model in R software (The R Foundation for Statistical Computing). Results. A total of 281 articles were retrieved; after applying the reading and exclusion criteria, 24 studies were included. The meta-analysis incorporated 24 studies from 12 countries, evaluating 2666 lesions with a biofilm prevalence of 68% (95% CI, 58%-79%; I² = 92%). A high prevalence was observed in Asian publications (73%; 95% CI, 62%-84%; I² = 98%), with of Staphylococcus aureus (71%; 95% CI, 51%-90%; I² = 98%) and Pseudomonas aeruginosa (65%; 95% CI, 47%-82%; I² = 98%) being the most common found in all publications. Conclusions. Despite the methodological heterogeneity of the studies included in this review, the findings indicate a high prevalence of biofilms in chronic wounds presented in the studies that made up the sample.
Introduction
Chronic wounds are lesions that do not follow the natural healing process, remaining open and stagnant at a certain stage of the healing process for a prolonged period, usually longer than 3 months. These wounds are often associated with underlying conditions such as diabetes, vascular disease, and prolonged difficulty mobilizing, which compromise the body’s ability to heal tissue effectively. Chronic wounds include pressure injuries, venous ulcers, and diabetes mellitus-related ulcers, each with specific characteristics related to their etiology.1
Inadequate and incomplete healing of these wounds can lead to serious complications such as infections and amputations. In addition, these complications require prolonged and complex care and compromise patients’ ability to perform daily activities, significantly decreasing their quality of life.2
Treating these lesions requires significant health care resources, involving specialized care, long periods of treatment, and recurrent hospitalizations.³ The high prevalence and challenges associated with treating these wounds highlight the need for effective prevention strategies and therapies worldwide.4
There are several intervening factors that delay healing; of these, the presence of biofilm stands out. Biofilms play an important role in wound chronicity, significantly hindering the healing process. The presence of biofilms in chronic wounds requires specific and more intensified therapeutic approaches because conventional treatment strategies, such as the administration of antibiotics, are often ineffective because of the resistance conferred by the protective matrix of the biofilm.5
Biofilms are structured communities of microorganisms that adhere to living or inanimate surfaces and are encased in a matrix of extracellular polymeric substances.6 This matrix—composed primarily of polysaccharides, proteins, and nucleic acids—protects the microorganisms from the harsh conditions of the external environment, such as the action of antimicrobials and the host’s immune system response.7
Techniques such as debridement, the application of topical antimicrobials, and the use of technologies that promote biofilm disruption—such as laser therapy and ultrasound—are being explored to combat these resistant microorganisms.8 The effective management of biofilms in chronic wounds is critical to improving clinical outcomes, reducing the burden of infection, and accelerating the healing process.
Thus, it is important to evaluate the prevalence of biofilm in chronic wounds in order to reveal its effect on lesions and communicate to health professionals, especially stomal therapy nurses, the need for complex care to combat it.
To this end, the objective of this systematic review with meta-analysis was to estimate the prevalence of biofilms in chronic wounds.
Of note, a review on this topic was published in 2017.9 The current study is an update that includes international research until September 2024.
Methods
This systematic review of prevalence studies was registered in the International Prospective Register of Systematic Reviews (PROSPERO) and carried out on March 30, 2024 (registered in PROSPERO (CRD420244526479). The review and meta-analysis were structured according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
The methodological stages of the Joanna Briggs Institute were followed for systematic prevalence reviews; this included the definition of the protocol title and review; definition of the review question; and definition of inclusion criteria and search strategy screening and selection of the study, evaluation and methodological quality, and extraction and synthesis of data.10
The research question that directed the study was constructed using the PCC mnemonic: Problem (biofilm), Concept (prevalence), Context (complex wounds). Studies that presented the prevalence of biofilm in humans, in any time frame without limitation of language and place of research, were included. Thus, no delimiter filters were used in any of the databases. Review studies, case studies, abstracts, dissertations, and theses were excluded.
Scopus (Elsevier), Web of Science (Clarivate), Embase (Elsevier), and MEDLINE/PubMed (National Institutes of Health) databases were searched on April 11, 2024, by 2 independent researchers in order to answer the following research question: What is the prevalence of biofilm in chronic wounds? For Scopus, Web of Science, and MEDLINE/PubMed, the Medical Subject Headings (MeSH) descriptors were used; Emtree descriptors were searched in Embase. A manual search of the list of references of the studies and gray literature was also conducted. Only articles from the databases were included because no studies were identified through the other searches.
All articles selected at that time were imported into Rayyan (Rayyan Systems, Inc.) for screening by the evaluators. To create the data strategy, the ECCCU search equation11 was used: extraction, conversion, combination, construction, and use. Due to the particularities of access to each database and in order to cover the largest number of studies, it was necessary to adjust the search strategy in certain research sources. The Box describes the strategy adopted in each of the databases.

After the initial articles were selected, the evaluators read the titles and abstracts to identify studies that were beyond the scope of the research. Studies that did not present primary data on the prevalence of biofilm in chronic wounds were excluded. This decision aimed to ensure that only research with specific quantitative information on the occurrence of biofilm was included, allowing for a more accurate and consistent analysis in the meta-analysis. Additionally, literature reviews and case studies were excluded.
Two independent evaluators participated in the selection of the studies. Any conflicts were later resolved by the 2 evaluators so that there was no need for a third evaluator.
A data collection instrument was used to capture the following information from each study: author and year of the research, total number of lesions evaluated, number of lesions with biofilm (prevalence), which bacteria was detected, level of biofilm, country where the research was developed, and methodological quality. The articles were read in full, and the data were collected and organized in an Excel spreadsheet (Microsoft).
The Newcastle-Ottawa Scale was adapted to evaluate the cross-sectional studies and consider their methodological quality. This instrument considers the following items for evaluation: sample representativeness, sample size, non-responder rate, how the outcome was evaluated, and statistical analyses performed. A score is given for each evaluated item, where the item that evaluates the outcome can reach up to 2 points, totaling from 0 to 6 points (very good studies: 6 points; good studies: 4-5 points; satisfactory studies: 3 points; unsatisfactory studies: < 2 points). To compare the groups in relation to methodological quality in the meta-analysis, up to 3 points were categorized as satisfactory quality and less than 3 as unsatisfactory.12
A meta-analysis was performed to analyze the prevalence of biofilm (primary outcome) using the CI and percentage rate. The Cochran’s Q test and I2 statistics were used to assess heterogeneity. When evaluating the homogeneity of the studies, high heterogeneity was considered when I2 was greater than 50 and Cochran’s Q P value was less than .10, thus applying the random effects model. Metaregression was performed to identify the cause of the heterogeneity of the studies.
Publication bias was assessed through visual inspection of the funnel plot as well as calculation of the Egger’s test, where the result to determine the absence of bias was indicated by a P value of greater than .05.13 For variables that could not be determined by the meta-analysis, qualitative synthesis was performed.
Subgroup analyses were performed for bacteria detected in the biofilm and for the continents where the research was carried out. The prevalence between moderate and high levels of biofilm in the lesions was also compared. Statistical analysis was performed using the R 2024.4 software (The R Foundation for Statistical Computing).
Results
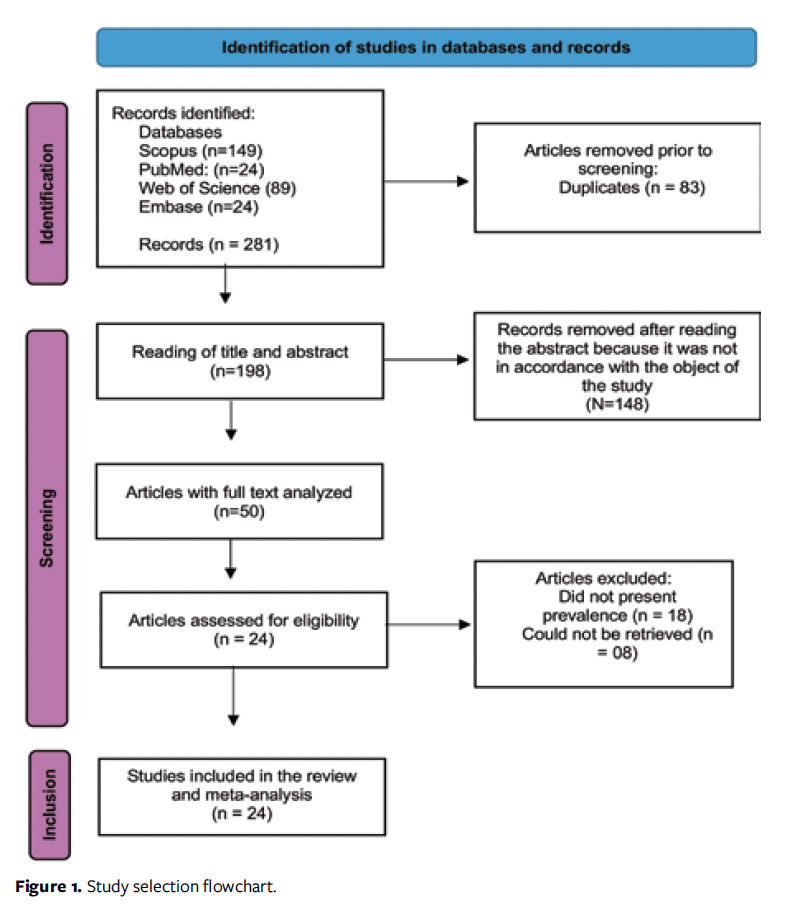
A total of 281 articles were identified by manual search in the databases. Duplicates (n = 83) were removed, resulting in 198 studies for evaluation. After analysis of the titles and abstracts, 148 additional studies were excluded for not meeting the objective of the research. The remaining 50 studies underwent full-text evaluation. Twenty-four studies from 12 countries were included in the meta-analysis (Figure 1).
The number of wound samples analyzed totaled 2666, ranging from 20 to 355 samples evaluated in each study, and the biofilm was isolated between 5 and 277 times in the lesions. Eleven studies also classified their samples as having biofilm-forming capacity; these were classified as strong, moderate, or weak biofilm producers.
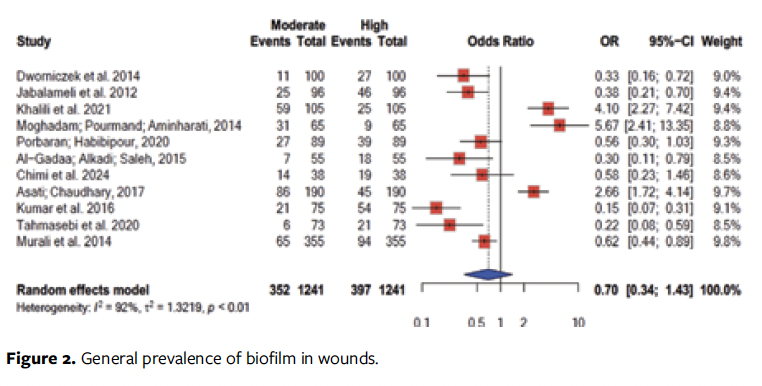
In the biofilm-level analysis performed in 11 studies, a higher chance of high biofilm levels (odds ratio [OR]: 0.70; 95% CI, 0.34-1.43) was recorded (Figure 2).
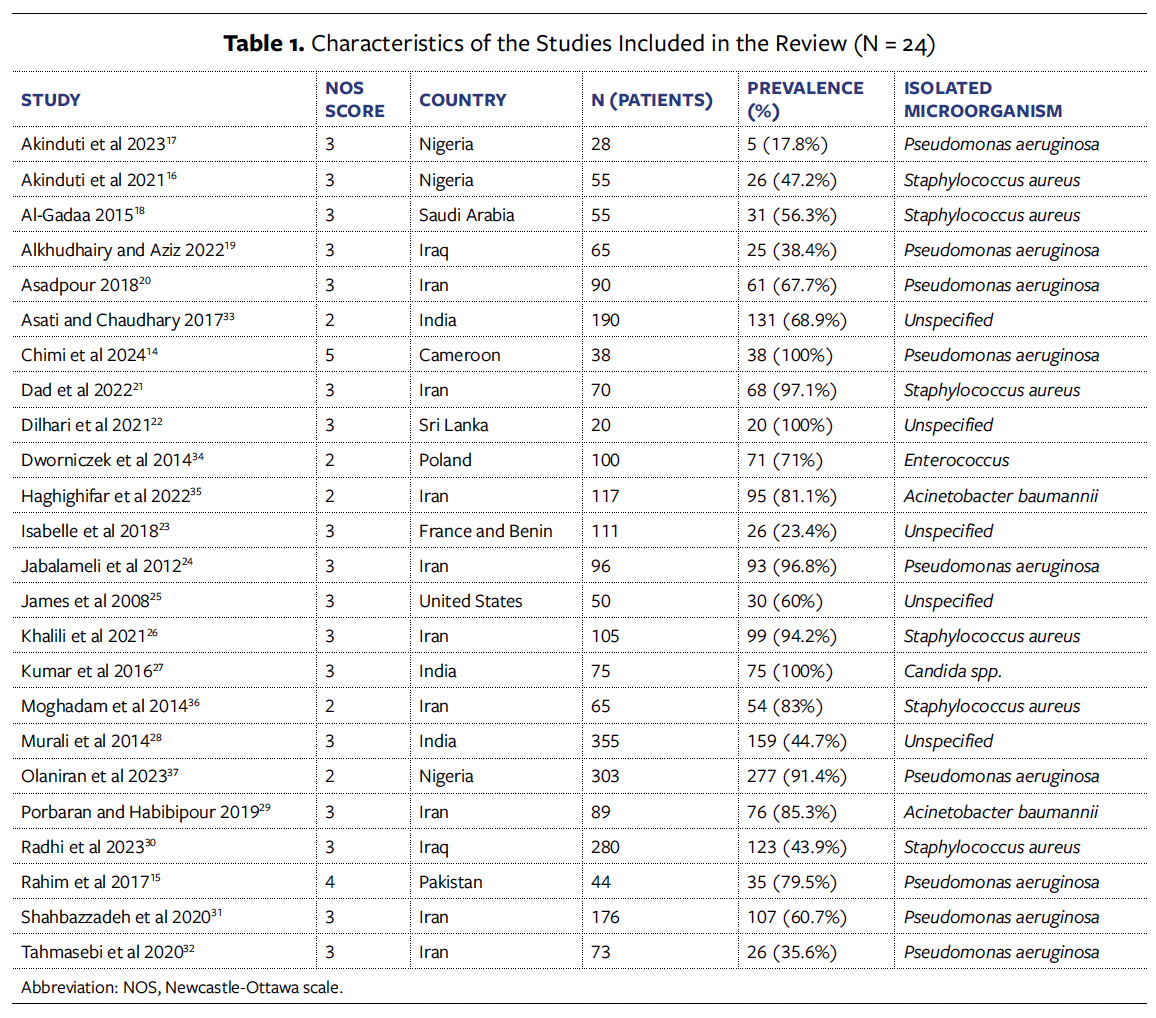
Of the studies that were included in the review, 2 presented good methodological quality,14,15 17 were classified as satisfactory,16-32 and 5 were classified as unsatisfactory.33-38 The synopses of the studies are shown in the Table.
The country with the most research on the prevalence of biofilm in wounds was Iran with 9 studies,20,21,24,26,29,31,32,35,36 followed by India27,28,33 and Nigeria16,17,37 with 3 studies each, and Iraq with 2.19,30 All other represented countries (the United States,25 Saudi Arabia,18 Poland,34 Sri Lanka,22 Pakistan,15 and Cameroon16) had 1 study each. A study that investigated the prevalence of biofilm in wounds in France and Benin was also included.23
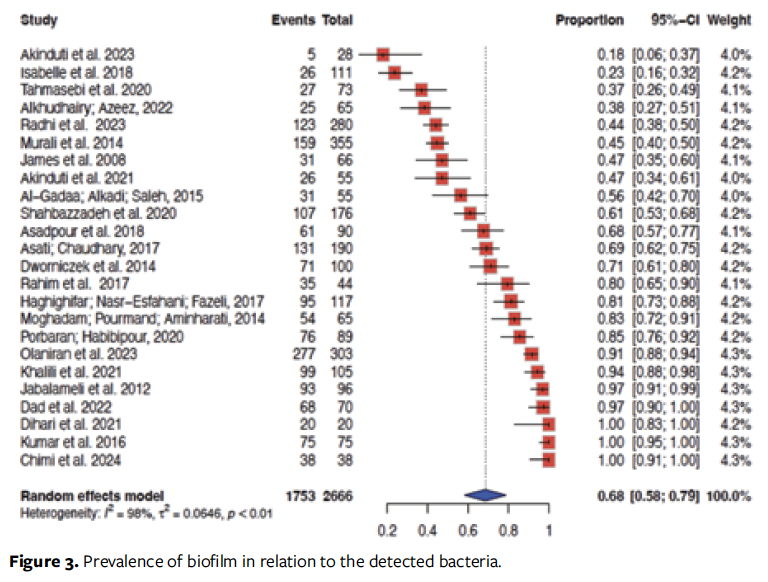
The overall biofilm prevalence was 68% (95% CI, 58%-79%), ranging from 23% to 100% (Figure 3).
Of the 24 articles, 9 identified the presence of biofilm formed by Pseudomonas aeruginosa (P aeruginosa)14,15,17,20,19,24,31,32,37 and 6 indicated Staphylococcus aureus (S aureus).16,18,26,30,36,21 Two studies investigated the presence of biofilm formed by Acinetobacter baumannii (A baumannii).29,35 Biofilms formed by Enterococcus spp34 and Candida spp27 were each searched in 1 study (Figure 4). The remaining studies did not look for biofilms formed by specific microorganisms.
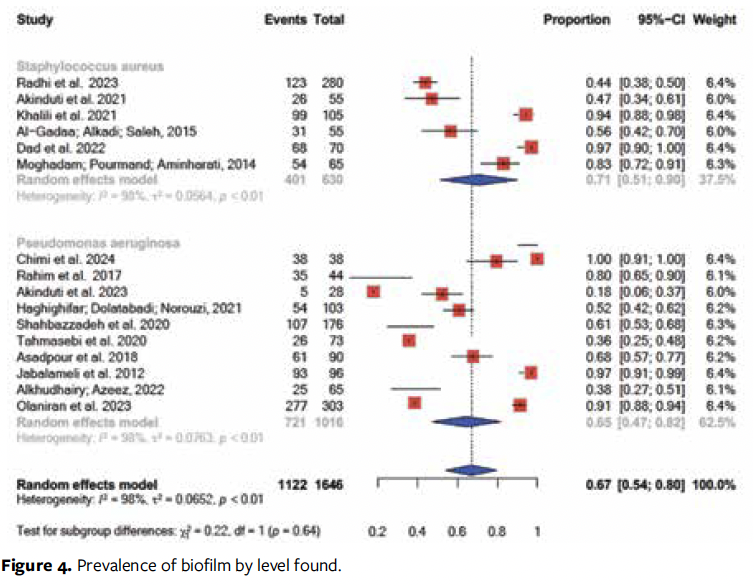
In a study carried out in Iran with strains of S aureus, 99 of 105 samples had biofilm formation, 25 of which were classified as strong biofilm-forming, 59 as moderate biofilm-forming, and 15 as weak biofilm-forming.26 In another study from Iran, this time with strains of A baumannii, 76 of the 89 isolates had biofilm, 39 of which were classified as strong, 27 as moderate, and 10 as weak biofilm-forming—these strains were multi-drug-resistant to antimicrobials.29 Another Iranian study examining the prevalence of biofilms formed by A baumannii found 95 positive samples out of 117.35
In a study in India, 159 out of 355 samples were positive for the presence of biofilm. The search was not performed on samples of a given microorganism, and the strains were multi-drug-resistant to antimicrobials. Of these strains, 94 were found to be strong biofilm-forming and 65 were found to be moderate biofilm-forming.28 In another study in India, 131 out of 190 samples showed the presence of the biofilm, with 45 being strong and 86 being moderate biofilm-forming.33
In a study that examined Candida spp samples in isolates collected from the feet of people with diabetes mellitus in India, all 75 samples were positive for biofilm; 54 were classified as strong to moderate biofilm-forming.27 A Polish study developed 100 samples of Enterococcus spp and found 71 to be biofilm-positive: 27 strong biofilm-forming, 11 moderate biofilm-forming, and 33 weak biofilm-forming.34
In the only study conducted with patients in more than 1 country, France and Benin, the mean age was 64 years and 26 of 111 samples were positive for biofilm.23 In the study from the United States, 30 of the 50 samples of chronic wounds showed the presence of biofilm.25 Furthermore, in the Sri Lankan study, 20 out of 20 samples were positive for the presence of biofilm.22
In Nigeria, studies looking for biofilms formed by P aeruginosa17 and S aureus16 showed the presence of biofilm in 5 of 28 and 26 of 55 samples, respectively. In another Nigerian study, out of 303 samples of P aeruginosa, 277 were positive for the presence of biofilm.37 A study from Pakistan showed the presence of P aeruginosa in 35 out of 44 samples.15 Of 65 samples with P aeruginosa collected from wounds in Iraq, 25 had biofilm.19 Another Iraqi study, this time concerning S aureus, found 123 out of 280 samples to have biofilm.30
In a study that collected P aeruginosa samples in Cameroon, 38 of 38 samples revealed the presence of biofilm. Of these, 19 were classified as strong biofilm-forming, 14 as moderate biofilm-producing, and 5 as weak biofilm-producing.14
The Saudi Arabian study included 55 samples of S aureus, 31 of which presented biofilm;18 of these, 18 were strong biofilm-forming, 7 were moderate biofilm-forming, and 6 were weak biofilm-forming.18
Several studies from Iran evaluated burn patients specifically. Dad et al showed that 68 out of 70 samples of S aureus had biofilm.21 Moghadam et al found 54 out of 65 isolated samples of S aureus to be positive for biofilm.36 Jabalameli et al showed the presence of biofilm in 93 of 96 samples of P aeruginosa; 46 were strong biofilm-formers, 25 moderate, and 22 weak.24 Asadpour found 61 of 90 samples of P aeruginosa to have biofilm.20 Radhi et al30 and Tahmasebi et al32 both examined P aeruginosa and found biofilm in 107 of 176 and 26 of 73 samples, respectively.
Subgroup analysis was also performed in relation to the continent where the studies were carried out (Figure 5). The meta-analysis showed significant differences in prevalence between the continents where the studies were published (P = .02), and the Asian continent showed the highest prevalence (73%; 95% CI, 62%-84%).
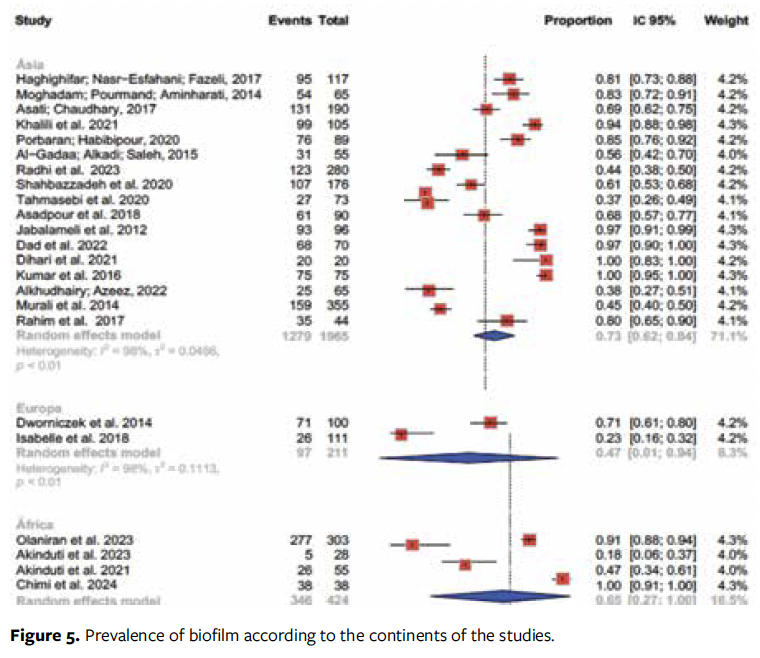
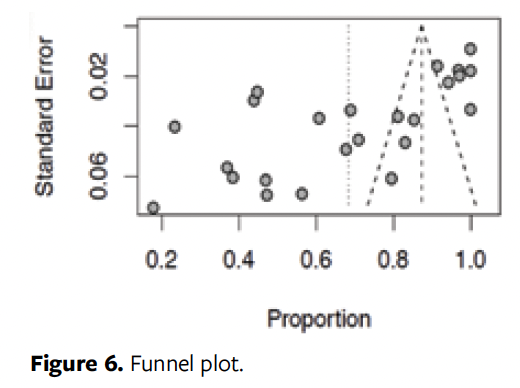
Figure 6 evaluates the risk of biases in the studies. The funnel plot shows asymmetry between the surveys confirmed by the Egger’s test (P = .000). Thus, the main meta-analysis showed a prevalence of 68%, but after the publication bias was verified, the model was adjusted by the trim-and-fill method, revealing that the actual prevalence reaches up to 98%, suggesting that the result of our main analysis is under publication bias. The meta-regression could not explain the causes of heterogeneity of the studies (P > .05).
Discussion
In this systematic review, the results indicate an overall prevalence of 68% of biofilm in chronic wounds. Thus, an intensive therapeutic approach is needed to prevent and treat this condition, as the presence of biofilm in wounds prolongs the healing time of chronic wounds, increasing health expenses and decreasing the patients’ quality of life.6
It should be noted that the time for biofilm development in the wound bed varies because of the different structures of each bacterium, so the stomal therapist must be aware of the clinical signs of the presence of biofilm in order to institute adequate therapy. Products based on Ag/Fe3O4 nanocomposites, propolys, tryptophan and enzymes, silver coatings, negative pressure therapy with silver, or polyhexamethylene instillation can reduce or remove biofilm.39
There was no significant difference between the types of bacteria isolated in the lesions, although S aureus had a higher prevalence (71%), corroborating a study that also shows a higher prevalence of S aureus than that of P aeruginosa.40
S aureus presents a significant challenge because of its large capacity for virulence-factor production and frequent resistance to available antibiotics.41 Nevertheless, P aeruginosa, another microorganism frequently isolated in the articles of this study, is also known for its intrinsic resistance to existing antimicrobials and for producing virulence factors.42
It is noteworthy that the studies in the review presented analyses of monospecies in the lesions, and that an in vitro study43 demonstrated the possibility of double bacterial installation, revealing an increase in the maturation of double biofilms, a 50% increase in tolerance to antibiotics, and a polymer matrix more resistant to drug penetration. This demonstrates that in-depth understanding of bacterial communities, as well as investment efforts in effective prevention and treatments, are increasingly emerging.43
Nevertheless, the potential of biofilm to negatively impact public health, regardless of the microorganism responsible for its formation, is remarkably relevant. This context is further intensified when considering the high levels of biofilm, which result in increased antimicrobial resistance, greater chronicity of wounds and infections, and increased virulence factors.12,44
The criteria used to determine what is considered to be a high level of biofilm may vary according to the context and method used for measurement. Microtiter plate assays can measure biofilm mass, and this was one of the main forms of measurement observed in the studies of the present research. By this method, levels of biofilm are considered to be high when there is a dense and extensive biofilm coverage on the tested surfaces.12 Clinically, high levels of biofilm are identified when infections are difficult to treat with standard antibiotic regimens, requiring higher doses or alternative therapies.45
Notably, there was a significant difference in the prevalence of biofilm between continents, with a remarkable 73% prevalence in chronic wounds on the Asian continent. The authors of the current study were not able to find any studies to justify such a finding; however, it is possible that the other continents are lacking comparable studies on biofilm prevalence. In addition, the difference may be the result of conditions such as socioeconomic status, demographics, race, and organization of health services, among others, that were not accounted for in this study and that directly impact the development and control of lesion infections. Lack of access to health services and individual perception can contribute to a delay in seeking care, facilitating complications in people with lesions.46
The methodological quality of the studies should also be considered. There was a small number of studies with very good or good methodological quality; however, the vast majority of studies were classified as satisfactory, followed by some as unsatisfactory. Additionally, one must consider the differences found between them.
This review confirms the high prevalence of biofilm in chronic wounds and presents a global overview of the research on this topic, thus stimulating the development of more research to understand and update the mechanisms of biofilm development for the purpose of producing effective therapies, as well as encouraging the stomal therapist to spare no effort in the correct treatment of chronic lesions. The inclusion of methodologically unsatisfactory studies has been mentioned as a limitation of this research.
Thus, more research—with satisfactory methodological quality—is needed to accurately define the prevalence of biofilm in wounds and guide the development of strategies aimed at its control and prevention. Traditional culture techniques may present a significant bias as a diagnostic tool, favoring the detection of microorganisms that grow easily, such as S aureus, while hindering the identification of hard-to-culture bacteria, such as anaerobes. Therefore, it is necessary to expand research to compare the results obtained with conventional cultures with those provided by high-resolution molecular techniques.
To care for wounds with biofilm, stomal therapist nurses must understand the nuances and action of the biofilm, including preventive management as well as antimicrobial and antibiofilm agents. Care for chronic wounds is a component of stomal therapy, a specialty exclusive to nurses who work in the care of people with wounds, incontinence, and ostomies.
Limitations
This study has limitations that should be considered. The geographic concentration of studies, particularly in Asia and the Middle East, limits the generalization of the results to other regions. Moreover, the absence of longitudinal studies prevents the assessment of causality between the presence of biofilm and chronic wounds. Publication bias, evidenced by the asymmetry of the funnel plot and Egger’s test, suggests that the actual prevalence of biofilm may be higher than reported.
Other limitations include the quality of the methods used, the prevalence found, and the results obtained from each study, which highlight the importance of investigating the methodological quality in review studies. When considering the methodological quality of the studies, this systematic review with meta-analysis is justified to make visible the heterogeneity of the results found.
Conclusion
This systematic review analyzed 24 articles on biofilm in chronic wounds, showing an overall prevalence of 68%. A considerable majority of the studies came from the Asian continent, and from Iran in particular. There was a higher prevalence of S aureus. It is important to note that the studies showed publication biases, so it is possible that the overall prevalence of biofilm may reach up to 98%.
Author & Publication Information
Authors: Manuela de Mendonça Figueirêdo Coelho, PhD1; Beatriz Moreira Alves Avelino, RN2; Beatriz Alves de Oliveira, MSN1; Mariana Araújo Rios, RN1; Fabiane do Amaral Gubert, PhD1; Mariana Cavalcante Martins, PhD1; Janaína Fonseca Victor Coutinho, PhD1; Paula Sacha Frota Nogueira, PhD1; Rachel Gabriel Bastos Barbosa, PhD1; and Viviane Mamede Vasconcelos Cavalcante, PhD1
Affiliations: 1Department of Nursing, Federal University of Ceará, Ceará, Brazil; 2Department of Nursing, Ceará State University, Ceará, Brazil.
Disclosures: The authors have no financial or other conflicts of interest to disclose.
Ethical Approval: The review and meta-analysis were structured according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Correspondence: Manuela de Mendonça Figueirêdo Coelho, Alexandre Baraúna Street 1115, Rodolfo Teófilo, 60430-160, Fortaleza, Ceará, Brazil; manumfc2003@yahoo.com.br
Manuscript Accepted: May 19, 2025
References
1. Pereira T de O, Lescano FA, Oliveira RAM de, Simões EAP. Artisanal subatmospheric therapy in the treatment of pressure injury. Brazilian Journal of Development. 2020;6(1):1560-1574. doi:10.34117/bjdv6n1-107
2. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27–32. doi:10.1016/j.jval.2017.07.007
3. Hayun Y, Yaacobi DS, Shachar T, Harats M, Grush AE, Olshinka A. Novel technologies in chronic wound care. Semin Plast Surg. 2022;36(2):75-82. doi:10.1055/s-0042-1749095
4. Gale HL, Staffa SJ, DePamphilis MA, Tsay S, Burns J, Sheridan R. Pediatric burn care for injury: outcomes by timing of referral using a U.U single-center retrospective cohort, 2005-2019. Pediatr Crit Care Med. 2024;25(12):1150-1158. doi:10.1097/PCC.0000000000003623
5. Ghoreishi FS, Roghanian R, Emtiazi G. Novel chronic wound healing by anti-biofilm peptides and protease. Adv Pharm Bull. 2022;12(3):424-436. doi:10.34172/apb.2022.047
6. Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563-575.
7. Jara CP, Silva JLG, Zanchetta FC, et al. Biofilm and chronic wound: reflections for nursing care. Revista Enfermagem Atual In Derme. 2017;81(19). doi:10.31011/reaid-2017-v.81-n.19-art.324
8. Stoffel JJ, Riedi PLK, Romdhane BH. A multimodel regime for evaluating effectiveness of antimicrobial wound care products in microbial biofilms. Wound Repair Regen. 2020;28(4):438-447. doi:10.1111/wrr.12806
9. Malone M, Bjarnsholt T, McBain AJ, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care. 2017;26(1):20-25. doi:10.12968/jowc.2017.26.1.20
10. Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z, eds. JBI Manual for Evidence Synthesis. JBI; 2024. doi:10.46658/JBIMES-24-01
11. Araújo WCO. Health information retrieval: construction, models and strategies. Convergências em Ciência da Informação. 2020;3(2):100-134. doi:10.33467/conci.v3i2.13447
12. Silva A, Silva V, López M, et al. Antimicrobial resistance, genetic lineages, and biofilm formation in Pseudomonas aeruginosa isolated from human infections: an emerging One Health concern. Antibiotics (Basel). 2023;12(8):1248. doi:10.3390/antibiotics12081248
13. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi:10.1136/bmj.d4002
14. Chimi LY, Noubom M, Bisso BN, Njateng GSS, Dzoyem JP. Biofilm formation, pyocyanin production, and antibiotic resistance profile of Pseudomonas aeruginosa isolates from wounds. Int J Microbiol. 2024;2024:1207536. doi:10.1155/2024/1207536
15. Rahim K, Saleha S, Basit A, et al. Pseudomonas aeruginosa as a powerful biofilm producer and positive action of amikacin against isolates from chronic wounds. Jundishapur Journal of Microbiology. 2017;10(10)e57564. doi:10.5812/jjm.57564
16. Akinduti AP, Osiyemi JA, Banjo TT, et al. Clonal diversity and spatial dissemination of multi-antibiotics resistant Staphylococcus aureus pathotypes in Southwest Nigeria. PLoS One. 2021;16(2):e0247013. doi:10.1371/journal.pone.0247013
17. Akinduti PA, George OW, Ohore HU, et al. Evaluation of efflux-mediated resistance and biofilm formation in virulent Pseudomonas aeruginosa associated with healthcare infections. Antibiotics (Basel). 2023;12(3):626. doi:10.3390/antibiotics12030626
18. Al-Gadaa AH, Alkadi A, Saleh E. Methicillin resistant Staphylococcus aureus and its biofilm in persistent diabetic foot ulcer in Qassim Region. Life Science Journal. 2015;12(2):1-8. doi:10.7537/marslsj120215.01
19. Alkhudhairy MK, Azeez MA. Detection of some virulence factors in extended spectrum lactamase producing-Pseudomonas aeruginosa isolated from wound infections in Al-Najaf City, Iraq. NeuroQuantology. 2022;20(15):1314–1329. doi:10.14704/NQ.2022.20.15.NQ88120
20. Asadpour L. Antimicrobial resistance, biofilm-forming ability and virulence potential of Pseudomonas aeruginosa isolated from burn patients in northern Iran. J Glob Antimicrob Resist. 2018;13:214-220. doi:10.1016/j.jgar.2018.01.018
21. Dad V, Ahmadrajabi R, Esfahani S, Saffari F. Comparative study of Staphylococcus aureus from burn patients and healthcare workers in a burn center, Yazd, Iran. Wien Med Wochenschr. 2022;172(11): 256–260. doi:10.1007/s10354-021-00863-5
22. Dilhari A, Weerasekera M, Gunasekara C, et al. Biofilm prevalence and microbial characterisation in chronic wounds in a Sri Lankan cohort. Lett Appl Microbiol. 2021;73(4):477-485. doi:10.1111/lam.13532
23. Isabelle F, Damien S, Florence R, et al. Occurrence and persistence of biofilms on cared chronic wounds: a large multicentric clinical study. Wound Medicine. 2018;23:28–34. doi:10.1016/j.wndm.2018.09.008
24. Jabalameli F, Mirsalehian A, Khoramian B, et al. Evaluation of biofilm production and characterization of genes encoding type III secretion system among Pseudomonas aeruginosa isolated from burn patients. Burns. 2012;38(8):1192–1197. doi:10.1016/j.burns.2012.07.030
25. James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16(1):37–44. doi:10.1111/j.1524-475X.2007.00321.x
26. Khalili H, Najar-Peerayeh S, Mahrooghi M, Mansouri P, Bakhshi B. Methicillin-resistant Staphylococcus aureus colonization of infectious and non-infectious skin and soft tissue lesions in patients in Tehran. BMC Microbiology. 2021;21(1):282. doi:10.1186/s12866-021-02340-w
27. Kumar D, Banerjee T, Chakravarty J, Singh SK, Dwivedi A, Tilak R. Identification, antifungal resistance profile, in vitro biofilm formation and ultrastructural characteristics of Candida species isolated from diabetic foot patients in Northern India. Indian J Med Microbiol. 2016;34(3):308. doi:10.4103/0255-0857.188320
28. Murali TS, Kavitha S, Spoorthi J, et al. Characteristics of microbial drug resistance and its correlates in chronic diabetic foot ulcer infections. J Med Microbiol. 2014;63(10):1377–1385. doi:10.1099/jmm.0.076034-0
29. Porbaran M, Habibipour R. Biofilm formation and β-lactamase enzymes: a synergism activity in Acinetobacter baumannii isolated from wound infection. J Adv Med Biomed Res. 2019;27(125):34–42. doi:10.30699/jambs.27.125.34
30. Radhi OA, Albandar I, Alqaseer K, Shnain WD. Selenium nanoparticles inhibit Staphylococcus aureus-induced nosocomial infection, cell death and biofilm formation. J Popul Ther Clin Pharmacol. 2023;30(4):367–378. doi:10.47750/jptcp.2023.30.04.036
31. Shahbazzadeh M, Moazamian E, Rafati A, Fardin M. Antimicrobial resistance pattern, genetic distribution of ESBL genes, biofilm-forming potential, and virulence potential of Pseudomonas aeruginosa isolated from the burn patients in Tehran Hospitals, Iran. Pan Afr Med J. 2020;36:233. doi:10.11604/pamj.2020.36.233.21815
32. Tahmasebi H, Dehbashi S, Alikhani MY, Porbaran M, Arabestani MR. Prevalence and molecular typing of metallo-β-lactamase-producing Pseudomonas aeruginosa with adhesion factors: a descriptive analysis of burn wounds isolates from Iran. Gene Reports. 2020;21:100853. doi:10.1016/j.genrep.2020.100853
33. Asati S, Chaudhary U. Prevalence of biofilm producing aerobic bacterial isolates in burn wound infections at a tertiary care hospital in northern India. Ann Burns Fire Disasters. 2017;30(1):39–42.
34. Dworniczek E, Piwowarczyk J, Seniuk A, Gościniak G. Enterococcus – virulence and susceptibility to photodynamic therapy of clinical isolates from Lower Silesia, Poland. Scand J Infect Dis. 2014;46(12):846–853. doi:10.3109/00365548.2014.952244
35. Haghighifar E, Nasr-Esfahani B, Fazeli H. Determination of biofilm formation ability and antibiotic resistance of Acinetobacter baumannii strains isolated from patients with burn wound infection. Journal of Isfahan Medical School. 2022;37(552):1280–1285. doi:10.22122/jims.v37i552.12561
36. Moghadam SO, Pourmand MR, Aminharati F. Biofilm formation and antimicrobial resistance in methicillin-resistant Staphylococcus aureus isolated from burn patients, Iran. J Infect Dev Ctries. 2014;8(12):1511–1517. doi:10.3855/jidc.5514
37. Olaniran OB, Donia A, Adeleke OE, Bokhari H. Prevalence of type III secretion system (T3SS) and biofilm development in genetically heterogeneous clinical isolates of Pseudomonas aeruginosa from Nigeria. Curr Microbiol. 2023;80(11):349. doi:10.1007/s00284-023-03467-x
38. Borges EL, Spira JAO, Amorim GL, Coelho ACSM. Biofilm formation cutaneous wounds and its behavior in the face of interventions: an integrative review. Rev Rene. 2022;23:e78112. doi:10.15253/2175-6783.20222378112
39. Makeri D, Odoki M, Eilu E, Agwu E. Update on prevalence and antimicrobial resistance of Staphylococcus aureus and Pseudomonas aeruginosa isolated from diabetic foot ulcers in Africa: a systematic review and meta-analysis. Bulletin of the National Research Centre. 2023;47(1).
40. Cutrim B da S. Avaliação cicatrizante de filmes de alginato contendo a lectina SteLL em feridas cutâneas frente a Staphylococcus aureus [Dissertação de Mestrado em Bioquímica e Fisiologia]. [Universidade Federal de Pernambuco]; 2023.
41. Tuon FF, Dantas LR, Suss PH, Tasca Ribeiro VS. Pathogenesis of the Pseudomonas aeruginosa biofilm: a review. Pathogens. 2022;11(3):300. doi:10.3390/pathogens11030300
42. Vestweber PK, Wächter J, Planz V, Jung N, Windbergs M. The interplay of Pseudomonas aeruginosa and Staphylococcus aureus in dual-species biofilms impacts development, antibiotic resistance and virulence of biofilms in in vitro wound infection models. PLoS One. 2024;19(5):e0304491. doi:10.1371/journal.pone.0304491
43. Yang S, Li X, Cang W, et al. Biofilm tolerance, resistance and infections increasing threat of public health. Microb Cell. 2023;10(11):233–247. doi:10.15698/mic2023.11.807
44. Patel N, Naidoo P, Candy G, Jann-Kruger C. Surgical infections at a regional hospital in Gauteng: reasons for delay to care and profile of pathology. South African Journal of Surgery (Online). 2019;57(1):30-36. doi:10.17159/2078-5151/2018/v56n4a2681
45. Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E, Rhoads D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS One. 2008;3(10):e3326. doi:10.1371/journal.pone.0003326
46. Zoltowski APC, Costa AB, Teixeira MAP, Koller SH. Qualidade metodológica das revisões sistemáticas em periódicos de psicologia brasileiros. Psicologia: teoria e pesquisa. 2014;30(1):97-104.
















