From Traditional to Single Use: The Evolution of Negative Pressure Wound Therapy as a Mechanism for Optimal Wound Management
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. The safety and efficacy of negative pressure wound therapy (NPWT) is well established. The technology has evolved to include 2 device categories: traditional NPWT (tNPWT) and single-use NPWT (sNPWT). Each mode has unique properties benefitting multiple aspects of wound care. Objective. To assess the proportion of tNPWT-treated wounds that could be amenable to sNPWT, thus determining optimal therapy. Materials and Methods. A de-identified dataset of wounds managed with tNPWT in outpatient clinics in the United States from 2006 through 2020 was analyzed to determine the proportion of wounds that could have been managed with sNPWT based on wound area, depth, and exudate volume, as well as sNPWT dressing size. Descriptive statistics were reported. Results. A total of 5040 wounds were analyzed. Ten wound types were identified, the most prevalent being surgical open wound (n = 2268 [45%]). All 8 commercially available sNPWT device dressing sizes, from 1 manufacturer, were included in the analysis. Overall, 3403 wounds (68%) would have been suitable to receive sNPWT instead of tNPWT at treatment commencement. Conclusion. The utilization of tNPWT is ideally positioned for large, deep, highly exuding wounds. However, by assessing a wound’s dimensions and exudate volume and type, a more appropriate NPWT device selection can be made; thus, ensuring the delivery of therapy with the most suitable device modality appropriate for the wound and patient while also maximizing resources.
Abbreviations: DFU, diabetic foot ulcer; EMR, electronic medical record; IFU, instructions for use; NPWT, negative pressure wound therapy; PI, pressure injury; sNPWT, single-use NPWT; tNPWT, traditional NPWT.
Background
NPWT devices have been used in clinical practice for the management of multiple open wound types since the 1990s, and they have been shown to be an efficacious therapy across inpatient and outpatient settings.1-3 Presently, there are 2 modes of NPWT delivery available to treat open wounds: tNPWT and sNPWT.1,3 tNPWT is commonly used to manage large, heavily exuding wounds by way of a canister-based system, and sNPWT is designed to manage low to moderate exudate volume and shallower wounds via a dressing-based, canister-less system. The tNPWT devices are reusable pumps that deliver subatmospheric pressure through either an open-cell foam or a gauze wound filler1,4 that is placed into the wound bed and secured with a film dressing. A rate of continuous or variable subatmospheric pressure is set by the clinician, usually between −50 mm Hg and −175 mm Hg1,3; this pressure is delivered via a suction port placed over a hole made in the film dressing securing the filler, by which exudate is removed into a disposable canister. In comparison, sNPWT devices encompass an integrated dressing and battery-powered pump that delivers a continuous, set level of −80 mm Hg subatmospheric pressure to the wound. In addition, sNPWT devices can be used with or without a foam or gauze wound filler, and the pump is small enough to fit into the palm of an adult hand and has a single on-off switch, enabling it to be worn discretely.4,5
It is generally accepted that the mode of action of NPWT supports an optimal wound environment through the removal of excess exudate, protection of the wound from external contaminants through a closed system, stimulation of granulation tissue, improved tissue perfusion, and reduction in edema.1,2,6 Uniquely, sNPWT has been shown to benefit the tissue surrounding the wound, the zone of injury, through compressive forces under and across the dressing area, potentially managing excess edema and interstitial fluid while also reducing periwound maceration and erythema compared with tNPWT.6
Dressing changes with each device can also depend on patient need and clinician assessment and should follow the recommended manufacturer IFU. tNPWT requires a dressing change every 48 to 72 hours,1,3 whereas sNPWT allows for undisturbed healing by reducing the removal of newly formed granulation tissue, as the dressing can stay in place for up to 7 days (if used without a filler). A reduction in dressing change frequency may also contribute to improved wound epithelialization.5
Both modes of delivery of NPWT provide similar clinical benefits to a wound, but having a choice of device allows clinicians flexibility based on wound and patient factors.3,6,7 Furthermore, tNPWT may be stepped across to sNPWT (or vice versa); for example, tNPWT may be suitable for a larger, heavily exuding wound until the overall volume of exudate reduces, at which time the wound may be transitioned to sNPWT.6 Most health care professionals determine which system to use on a case-by-case basis, which can lead to inconsistency and variation in when to use NPWT, which system to use, and, where appropriate, when to transition between the 2 devices.5 Despite its widespread adoption there are often further challenges associated with NPWT use, including a potential negative effect on patient quality of life; lack of staff experience and knowledge, time, and resources to perform dressing changes; and delayed hospital discharge.4,5 Evidence suggests that patients may discontinue tNPWT due to a detrimental effect on their quality of life because tNPWT devices are large and cumbersome, limiting patient mobility,6 particularly when receiving NPWT at home.3,8 sNPWT offers a lightweight, portable alternative to tNPWT due to its canister-less, single-use design, while still offering optimal clinical performance.6 A step-across approach from tNPWT to sNPWT, as clinically appropriate, may reduce the number of patients discontinuing therapy, and therefore increase concordance. It is also important to consider how NPWT delivery can be affected if limited devices are available. An example scenario would be an outpatient clinic having only 2 remaining tNPWT pumps available for use, but 3 patients present with open wounds that require NPWT. It is then critical that wounds are assessed for appropriate NPWT device use, where tNPWT and sNPWT devices need to be equally considered so as not to inhibit patient care and gold standard treatment.
In response to the aforementioned challenges, an international consensus panel of experts in wound care and NPWT from Canada, Spain, the United States, and the United Kingdom convened in 2020 to address how and when to use NPWT, and when to transition between tNPWT and sNPWT. The panel of experts developed and aligned upon a NPWT clinical decision tree (Figure 1) and 10 key evidence-based practice recommendations to aid in decision-making regarding NPWT systems, which in turn may improve therapy implementation, access to care, and patient quality of life while improving operational and financial efficiencies for health care providers.5 The clinical decision tree is an algorithm to aid in determining when to select tNPWT or sNPWT based on wound size, depth, and exudate levels. The panel’s recommendations also allow for further considerations, including patient satisfaction and quality of life, factors related to the care setting, economic factors, and NPWT device-related factors. Through case presentations, Loney et al7 demonstrated the clinical application of the consensus panel recommendations and the decision-making tree to optimize NPWT delivery and patient outcomes within inpatient and outpatient settings.
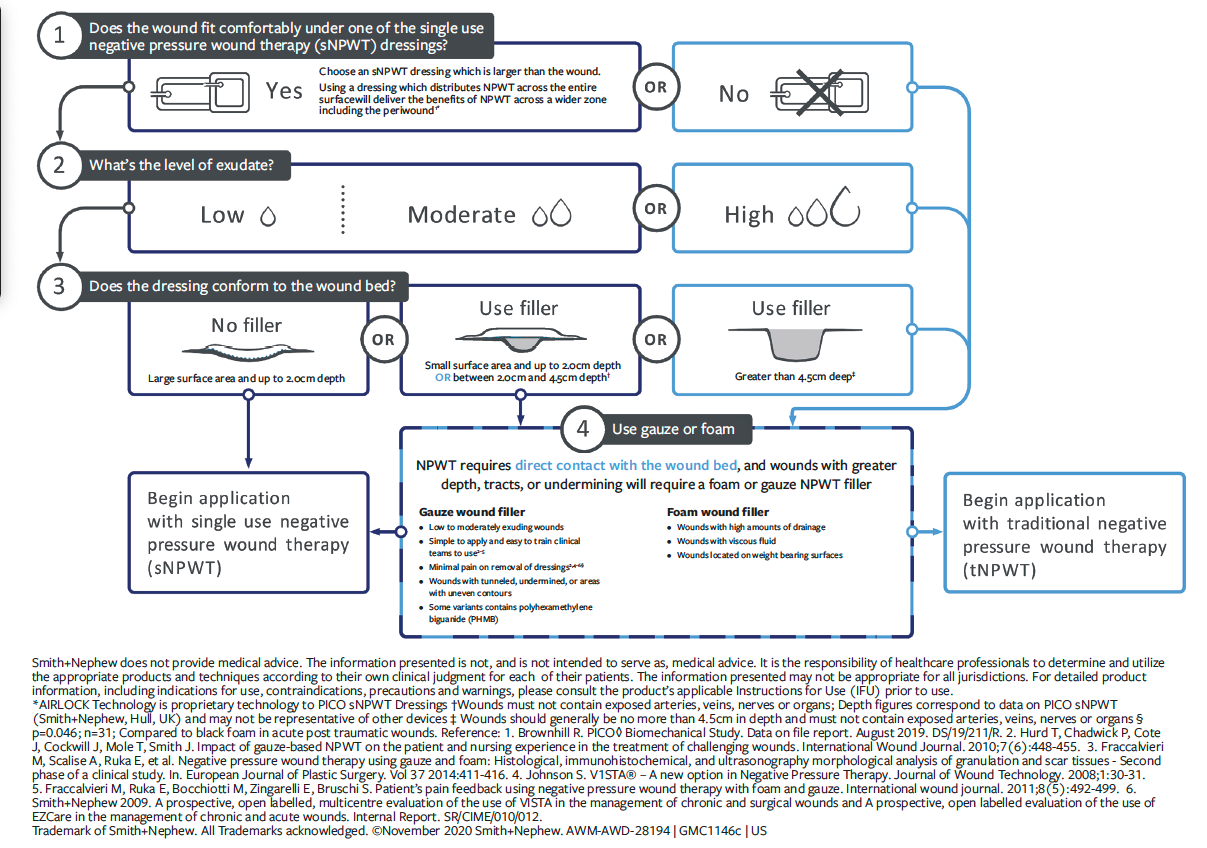
Although clinical guidelines and evidence support the use of sNPWT for open wounds with low to moderate exudate and a depth of up to 4.5 cm,9 sNPWT is still widely underutilized in clinical practice across all care settings. An example of this is a cost-minimization analysis published in 2018 of 1414 open wounds treated with tNPWT across long-term care settings, which found that 1249 (88.3%) of wounds met the criteria for treatment with sNPWT.10 This finding was based on wound exudate volume and dimensions. The authors of that article further demonstrated that the use of sNPWT instead of tNPWT would have resulted in daily treatment cost savings per patient of US $55 and significant total treatment cost savings per patient of US $1586.10
The aim of this analysis was to assess the proportion of wounds treated with tNPWT from an outpatient wound clinic electronic medical record (EMR) dataset that could be amenable to sNPWT, thus determining optimal treatment and device modality.
Materials and Methods
Data were purchased from an EMR vendor (Net Health), specifically for patients in the United States undergoing tNPWT in an outpatient setting. A retrospective, descriptive analysis was conducted on a dataset of wounds treated using a specific proprietary tNPWT device (V.A.C. NPWT system; 3M). All patients treated with that particular tNPWT device from 2006 through 2020 were included in the dataset. All data received were de-identified and in alignment with all local laws and regulations. Institutional review board approval was not sought because this analysis was conducted on commercially available, retrospective, routinely captured data to determine the prevalence of wounds that could have an alternative treatment modality, through the analysis of wound-specific data points.
All analyses were conducted using R software (version 4.0.3; The R Project for Statistical Computing) within RStudio (version 1.3.1093). Data tables for the tNPWT device were imported into RStudio. Lengths, informants, formats, and type of variables were preserved as in the data dictionary provided by the EMR vendor. Encounters for the named tNPWT device within the purchased dataset were identified by a pattern match algorithm searching the tables with the corresponding fields: TreatmentNotes: ProductName; PhysicianOrders: Name, Description; OtherProcedures: ProcedureName, Notes; SkinSubstitutes: Product; and NPWT: ProductGroup. Once all wounds treated with the tNPWT device were identified, each patient was screened for inclusion in the analysis using the criteria detailed in Table 1.
The inclusion of wounds in the analysis required the lengths and widths to be greater than the device-specific first quantile, which was 21 mm and 16 mm, respectively. In addition, the wounds included required completed data on depth, exudate type, and volume, and at least 2 documented treatment periods on a per-patient basis. This selection criterion enabled the following descriptive variables to be reported: wound type, anatomical location, wound dimensions, exudate volume and type, number of days treated with NPWT, and cessation of tNPWT. The reporting of the descriptive variables enabled the wounds treated with the tNPWT device to be assessed for treatment suitability by an sNPWT device (PICO 7 Single Use Negative Pressure Wound Therapy System; Smith and Nephew). The sNPWT device is indicated for use in the following wound types in the United States: closed surgical incisions, chronic wounds, acute wounds, traumatic wounds, subacute and dehisced wounds, partial-thickness burns, venous leg ulcers, diabetic ulcers, pressure ulcers, and flaps and grafts. The indications for use for the aforementioned tNPWT device are nearly the same, except that the tNPWT device is not indicated for use on closed surgical incisions.
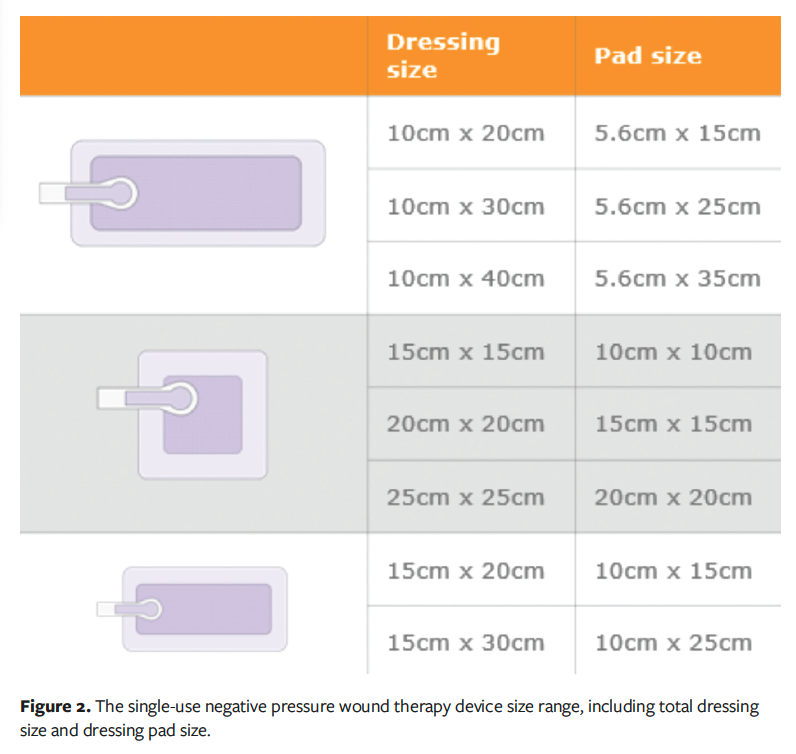
Using the sNPWT IFU11; consensus guidelines in the literature5; and the wound depth, exudate volume, and dressing size range of the sNPWT device, the proportion of wounds that could have received sNPWT rather than tNPWT was calculated. The dressing size ranges of the sNPWT device by total dressing size and dressing pad size are illustrated in Figure 2. The total dressing size and pad size are stated individually as per the device IFU; the wound must fit within the dimensions of the pad size. To further ensure accuracy of this descriptive analysis, wound depth was limited to 4.5 cm, as per the US IFU: “wounds treated with the sNPWT device should generally be no more than 4.5 cm in depth.”
Results
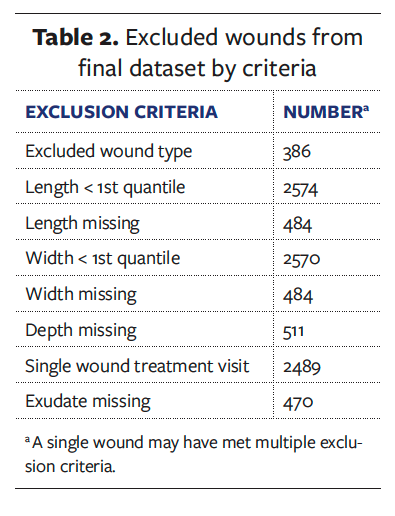
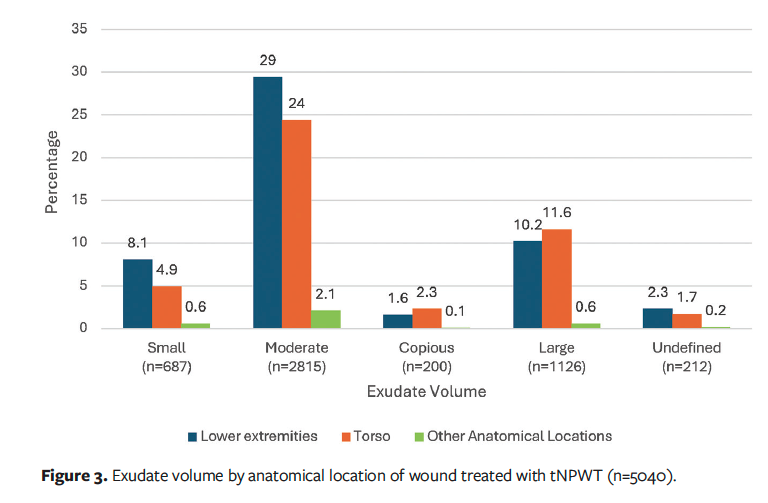
The de-identified dataset consisted of 10996 wounds treated with the tNPWT device. A total of 5956 wounds were excluded from the dataset. Table 2 details the number of wounds excluded per criteria. A wound may have been excluded from the dataset due to meeting multiple exclusion criteria. After screening the dataset for the inclusion and exclusion criteria, a final dataset of 5040 wounds was analyzed. Table 3 details the wound types and anatomical locations. A multitude of open wound types were identified, with the most prevalent being surgical open wounds, PI, and DFU. Concerning anatomical location of the wounds identified, most were located in the lower extremities (51.7%), with a further 44.8% occurring on the torso. The anatomical location does correlate to the most prevalent wound types in the dataset; open surgical wounds commonly occur in the abdomen, whereas PIs (eg, heel) and DFU occur in the lower extremities. These are representative of the typical wounds treated in outpatient settings.
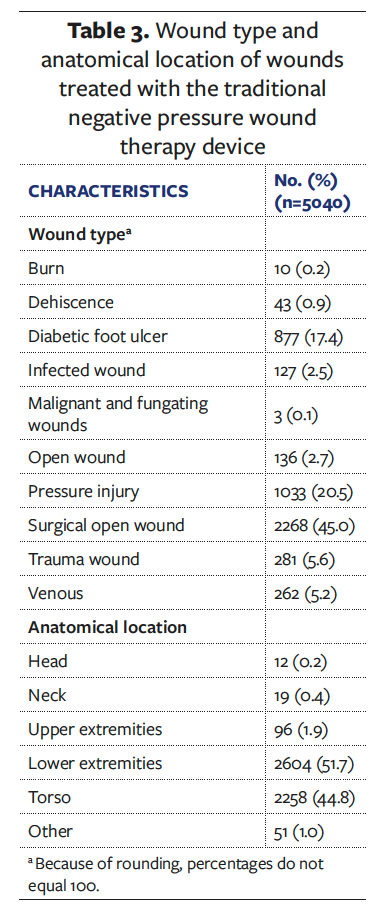
With a specific focus on the anatomical locations with the highest number of wound types, the volume of exudate was assessed and reported in Figure 3.
Exudate volume is categorized as low, moderate, and high. The volume and type of exudate are among the key variables that drive clinical decision-making concerning the type of NPWT device to use, whether a canister-based or a canister-less–based system. As the data reported in Figure 3 show, in treatment with a tNPWT device using a canister, all types of exudate volume are present, as a key differentiator in tNPWT is that it can manage low to high exudate. In comparison, a canister-less sNPWT device is indicated for low to moderate exudate; thus, a wound exhibiting heavy exudate would not be suitable for sNPWT. The categorization of exudate volume within the proprietary EMR from which the study data were purchased is more granular than the categories “low,” “medium,” and “high,” which is reflected in the dataset; however, there is a clear distinction that 56% of the wounds had moderate exudate volume, indicating that these wounds would be suitable for either a tNPWT or an sNPWT device.
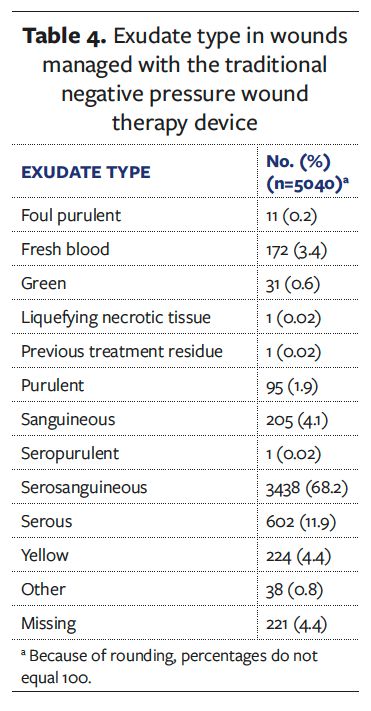
Furthermore, regarding exudate volume, the type of exudate in terms of its viscosity is another important factor to consider when managing wounds with NPWT. Table 4 describes the exudate types present during the tNPWT treatment within the dataset. Serous, serosanguineous, and sanguineous exudate account for 84% of the exudate types recorded. These exudate types tend to be low viscosity and present a low risk to NPWT delivery and the ability to remove the fluid to a canister or a dressing. However, it is important to consider that a small proportion of the wounds treated with the tNPWT device exhibited exudate types (eg, fresh blood [3.4%]) that are a contraindication to all NPWT and would require discontinuation of the treatment. The presence of purulent exudate warrants particular consideration because it has a higher viscosity and may require higher rates of NPWT to enable effective removal from the wound, while also potentially causing blockages within the NPWT delivery port. In the presence of purulent exudate, tNPWT with instillation and dwell, antimicrobial dressings, or debridement options may be required before or in conjunction with sNPWT.
When considering the use of an sNPWT device, the dimensions of the wound must be taken into account due to the range of dressing sizes available. The sNPWT device has 8 dressing sizes that are commercially available in the United States (Figure 2), with a maximum dressing size of 25 cm × 25 cm (250 mm × 250 mm). This is a key differentiator between tNPWT and sNPWT because with the use of a foam or gauze filler, tNPWT can be conformed to most wound sizes and is not generally limited to a maximum dressing size. The dimensions of all wounds in the dataset by anatomical location are reported in Table 5. The maximum wound area that would be suitable for sNPWT is 400 cm2 (4000 mm2). In the United States, the sNPWT device is recommended to be used in wounds with a maximum depth of 4.5 cm (450 mm), as stated in the device IFU.
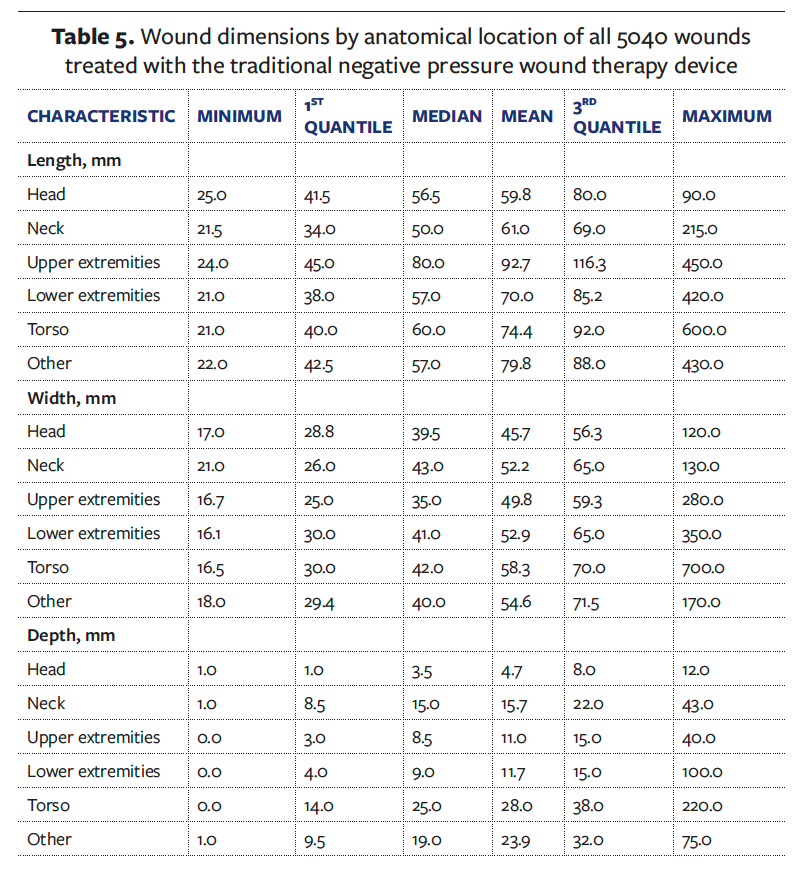
Table 6 details, by wound type and sNPWT dressing size, the number and percentage of wounds that would be amenable to sNPWT device use. These values were calculated by assessing the wound dimension, exudate volume (low to moderate), and depth. Overall, 68% of the wounds in the outpatient dataset (n = 3403) would have been suitable for sNPWT device treatment.
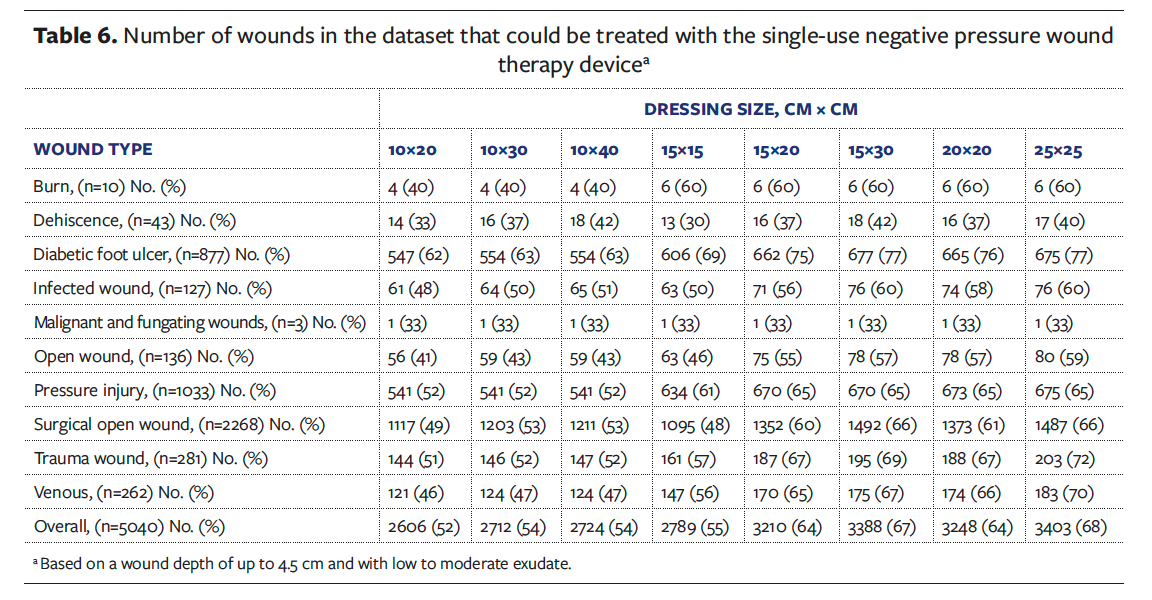
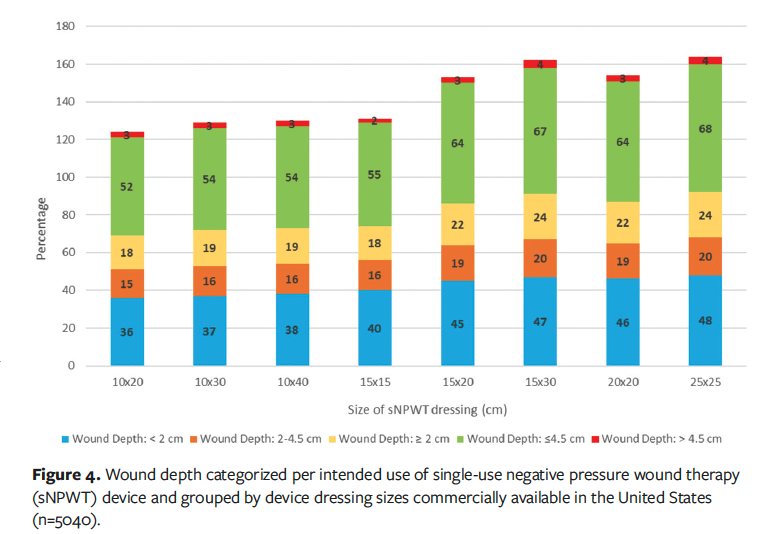
Further analysis was conducted that enabled a breakdown of the wounds by depth categorization. The use of sNPWT with and without filler is guided by wound depth: a depth less than or equal to 2 cm may not require use of a filler, a depth of 2 cm to 4.5 cm does require the use of a filler, and a depth greater than 4.5 cm is not recommended for use in the United States. The proportion of wounds present within the dataset by depth categorization is presented in Figure 4. Wounds with a depth greater than 4.5 cm account for 4% (n = 193) of the total wounds. Therefore, the main driver for consideration of the use of sNPWT within this dataset is wound area and exudate volume.
Discussion
The objective of the reported analysis was to determine the number of wounds treated with tNPWT in an outpatient setting that could be amenable and suitable for sNPWT device use, using data from across the United States. This is an important consideration, because NPWT is widely accepted as a beneficial therapy and often is selected to promote closure of acute and chronic wounds.1,2,5 Although both delivery modes offer similar benefits in terms of wound closure, evidence suggests that for venous leg ulcers and DFUs, significantly higher wound closure rates can be achieved with sNPWT than with tNPWT.12,13 These wound types often present with lower levels of exudate and could be treated with sNPWT if clinicians and patients had the ability to choose between NPWT devices and thus assess wounds for optimal device use. Furthermore, cost-effectiveness analysis between tNPWT and sNPWT devices demonstrated a cost saving with sNPWT of US $8483 and US $7325 in DFUs and VLUs, respectively, over a 12-week period.12 Kirsner et al12 provides a comprehensive assessment of multiple wound types treated in an outpatient environment that could have received an alternative NPWT device without affecting wound management.
A thorough wound assessment is the key first step in making a clinically driven decision concerning the optimal NPWT device for the wound, especially given the many factors clinicians need to consider, including the needs of the patient, available resources, and the cost of therapy. The international consensus guidelines acknowledged the wider aspects of decision-making in NPWT and detailed 10 statements for consideration when adopting it.5 These statements are supported by a clinical decision-making tree5 (Figure 1) that guides the clinician on which NPWT delivery system should be considered according to exudate levels and wound dimensions. With this approach, a clinician can ensure delivery of therapy with a suitable device modality appropriate for the wound and patient, while also maximizing resources.
Resources are finite within health care systems. These systems do not have an unending supply of NPWT devices, which is another reason choice of NPWT device is important; a clinician does not want to be in a position in which a patient needs NPWT and there are no available devices. For example, if only 1 tNPWT device were available, but sNPWT devices were readily available and 5 patients presented with open wounds, how does the clinician choose who receives tNPWT? What would happen if a sixth patient presented with a deep, heavily exuding wound? Therefore, by assessing the wounds for NPWT device, a choice does not have to be made. Effective wound assessment enables tNPWT and sNPWT modes to be prescribed to the appropriate patients with the most suitable wounds, thus enabling effective treatment for all. Figure 5 provides a detailed explanation of NPWT device positioning and how this can be effectively put into place within a health care setting to ensure resources are used efficiently. Logistically, therefore, a patient with a low- to moderate-exuding wound (<400 cm2) can benefit from an sNPWT device that may offer more patient convenience, whereas a patient with a very deep (>4.5 cm) and heavily exuding wound would require tNPWT.
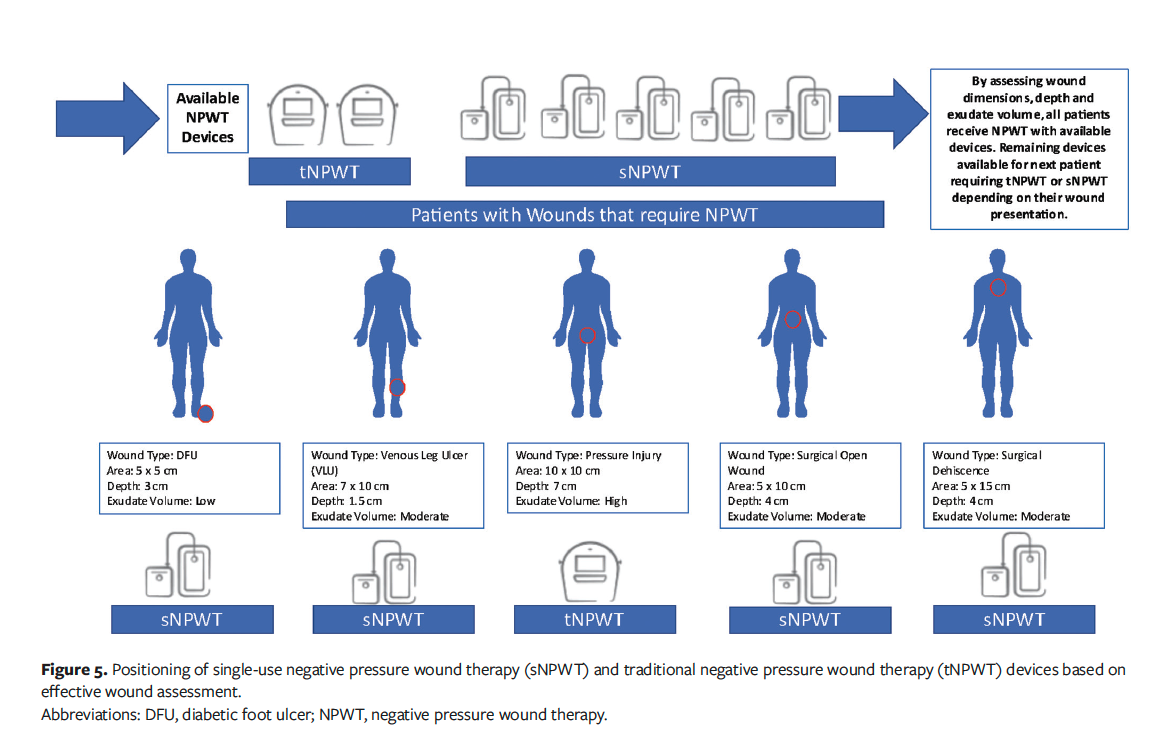
Further considering resource availability, sNPWT addresses some of the practical limitations of tNPWT for wounds for which either tNPWT or sNPWT would be suitable, such as increased portability, small size, less noise, fewer dressing changes (if applicable), and increased patient comfort.14 The sNPWT device on which this analysis is based is small, lightweight, and battery-powered and can be attached to a waistband or possibly placed in a pocket, which allows greater patient freedom. For wounds with a depth of less than 2 cm that may not require a filler, the sNPWT dressing may remain in place for up to 7 days; in comparison, a tNPWT with filler requires a dressing change every 48 hours to 72 hours.1 Therefore, sNPWT can reduce the number of dressing changes and associated resource costs. In the dataset used in the present analysis, 2396 of the 5040 wounds (48%) would have met this criterion. Fewer dressing changes not only reduces cost but enables undisturbed wound healing, maintenance of a moist wound environment, and optimization of the healing response by promotion of tissue granulation and epithelialization. Ultimately, this may reduce the time to reach treatment goals.15
Limitations
A key limitation of this study is the retrospective and descriptive nature of the analysis. Using multiple variables, the authors theorized to determine the wounds in the dataset that could benefit from sNPWT as opposed to the tNPWT that was received. It must be noted that NPWT, whether a traditional or single-use device, is an optimal management for open wounds; thus, time to healing was not a focused outcome of this analysis. A natural next step for this research would be to prospectively assess wounds for tNPWT or sNPWT use and complete longitudinal follow-up to determine time to healing, which could include a comparison of healing rates between each device mode, as well as cost-effectiveness. Furthermore, along with assessing clinical efficacy, future research should compare length of treatment between tNPWT and sNPWT and the optimal step-across timing (switching from tNPWT to sNPWT based on wound characteristics) between the devices, evaluate reimbursement status by treatment setting, and compare overall device sustainability between reusable tNPWT pumps compared with sNPWT devices.
Conclusion
he evolution of NPWT from traditional canister-based systems to single-use canister-less systems has enabled a multifactorial approach to wound management. The present analysis demonstrated that 68% of wounds treated with tNPWT are amenable to and could be treated with sNPWT. This allows clinicians and patients the ability to choose the most appropriate NPWT modality for their wound. By effectively assessing a wound, the optimal NPWT device can be utilized.
Author and Public Information
Authors: Rodney Lindsay, MD¹; Catherine H. McCarthy, RGN2; Jiunn-Ru (Angela) Lin, MSc3; Leo Nherera, PhD3; and Julie M. Murdoch, PhD2
Acknowledgments: 1Comprehensive Wound Center, Carrollton Regional Medical Center, Carrollton, TX, US; ²Global Clinical and Medical Affairs, Smith and Nephew, Watford, UK; 3Health Economics and Outcomes Research Data Analytics, Smith and Nephew, Fort Worth, TX, US
Acknowledgments: The authors would also like to thank Ashley Hudson, RN, BSN, NSWOC, IIWCC, for her contributions to manuscript development.
ORCID: Murdoch, 0000-0002-9903-0925
Ethical Approval: Institutional review board approval was not sought because this analysis was conducted on commercially available, retrospective, routinely captured data to determine the prevalence of wounds that could have an alternative treatment modality, through the analysis of wound-specific data points.
Disclosure: R.L. received a consultancy fee for his time participating in the development and writing of this manuscript. J.M.M., L.N., J.R.L., and C.H.McC. are employees of Smith and Nephew.
Correspondence: Julie M. Murdoch, PhD; Global Clinical and Medical Affairs, Smith and Nephew, Building 5, Hatters Lane, Croxley Park, Watford, WD18 8YE, United Kingdom; Julie.Murdoch@smith-nephew.com
Manuscript Accepted: February 5, 2025
References
1. Apelqvist J, Willy C, Fagerdahl AM, et al. EWMA document: negative pressure wound therapy. J Wound Care. 2017;26(Sup3):S1-S154. doi:10.129/jowc.2017.26.Sup3.S1
2. Birke-Sorensen H, Malmsjo M, Rome P, et al. Evidence-based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer)--steps towards an international consensus. J Plast Reconstr Aesthet Surg. 2011;64 Suppl:S1-16. doi:10.1016/j.bjps.2011.06.001
3. Apelqvist J, Fagerdahl AM, Teot L, Willy C. Negative pressure wound therapy: an update for clinicians and outpatient care givers. Journal of Wound Management. 2024;25(2 Sup 1):S1-S56. doi:10.35279/jowm2024.25.02.sup01
4. Kirsner RS, Hurd T. Assessing the need for negative pressure wound therapy utilization guidelines: an overview of the challenges with providing optimal care. Wounds. 2020;32(12):328-333.
5. Hurd T, Kirsner RS, Sancho-Insenser JJ, et al. International consensus panel recommendations for the optimization of traditional and single-use negative pressure wound therapy in the treatment of acute and chronic wounds. Wounds. 2021;33(suppl 2):S1-S11.
6. Brownhill VR, Huddleston E, Bell A, et al. Pre-clinical assessment of single-use negative pressure wound therapy during in vivo porcine wound healing. Adv Wound Care (New Rochelle). 2021;10(7):345-356. doi:10.1089/wound.2020.1218
7. Loney A, Diaz-Garcia RJ, Murdoch JM, Spitzer M. Utilizing international consensus panel recommendations and a clinical decision tree to guide selection of negative pressure wound therapy. Wounds. 2023;35(7):E209-E217. doi:10.25270/wnds/22085
8. Upton D, Andrews A. Negative pressure wound therapy: improving the patient experience Part 2 of 3. J Wound Care. 2013;22(11):582-591. doi:10.12968/jowc.2013.22.11.582
9. Hurd T, Gilchrist B, Ganguly A, Huddleston E. Single-use negative pressure wound therapy (sNPWT) in the community management of chronic open wounds deeper than 2cm. Poster presented at the Joint EWMA-Journées Cicatrisations Virtual Conference; October 26-27, 2021.
10. Adeyemi A, Waycaster C. Cost-minimization analysis of negative pressure wound therapy in long-term care facilities. Wounds. 2018;30(2):E13-E15.
11. Smith & Nephew Sa. PICO Single Use Negative Pressure Wound Therapy System Instructions for Use. 2022. Accessed March 18, 2024. https://www.possiblewithpico.com/instructions-use
12. Kirsner RS, Delhougne G, Searle RJ. A cost-effectiveness analysis comparing single-use and traditional negative pressure wound therapy to treat chronic venous and diabetic foot ulcers. Wound Manag Prev. 2020;66(3):30-36.
13. Kirsner RS, Zimnitsky D, Robinson M. A prospective, randomized, controlled clinical study on the effectiveness of a single-use negative pressure wound therapy system, compared to traditional negative pressure wound therapy in the treatment of diabetic ulcers of the lower extremities. Wound Repair Regen. 2021;29(6):908-911. doi:10.1111/wrr.12966
14. Hampton J. Providing cost-effective treatment of hard-to-heal wounds in the community through use of NPWT. Br J Community Nurs. 2015;Suppl Community Wound Care:S14, S16-S20. doi:10.12968/bjcn.2015.20.Sup6.S14
15. Rippon MG, Rogers AA, Ousey K, Atkin L, Williams K. The importance of periwound skin in wound healing: an overview of the evidence. J Wound Care. 2022;31(8):648-659. doi:10.12968/jowc.2022.31.8.648
















