An Overview and Survey of US Food and Drug Administration–Registered Wound Imaging Devices Capable of Determining Percentage Area Reduction and/or Percentage Volume Reduction
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. A key element in achieving high-quality research lies in the use of validated tools for effective data collection. To enhance this process, the authors have created comprehensive reporting guidelines for these essential tools. Objective. As members of the Wound Care Collaborative Community (WCCC) Tools Work Group, to identify tools capable of measuring percentage area reduction (PAR) and/or percentage volume reduction (PVR) and to assess the various features associated with these wound imaging devices. Methods. Data from publicly available databases and surveys of manufacturers meeting the inclusion criteria were collected in the fourth quarter of 2023 and the second quarter of 2024. The inclusion criteria were medical devices with the US Food and Drug Administration (FDA) FXN (Tape, Camera, Surgical) product code classification capable of length and width wound measurements currently marketed by FDA-listed establishments. Results. Thirteen FDA-registered establishments with 14 wound imaging devices met the inclusion/exclusion criteria. Representatives from 10 of those establishments engaged in the survey process by completing and returning their WCCC questionnaires. Seven tables were created to summarize the findings across the devices. Conclusion. More wound imaging devices measure PAR than PVR, and most utilize the perimeter alone or in conjunction with square surface area and/or oval or other shapes for area calculations. While variations in length or width (up to 5%), and area (up to 10%) may occur with the device calculations, these variations are less than is seen with ruler measurements. Several imaging devices incorporate methods to correct for skew, have sophisticated 3-dimensional modeling, and/or use reference markers to enhance overall accuracy and reliability, supporting their measurements as reliable indicators of wound trajectory.
Abbreviations: 2D, 2-dimensional; 3D, 3-dimensional; FDA, US Food and Drug Administration; GUDID, Global Unique Device Identification Database; PAR, percentage area reduction; PVR, percentage volume reduction; TWG, Tools Work Group; WCCC, Wound Care Collaborative Community.
Successful wound management requires a holistic approach, starting with a thorough patient history, physical examination, and complete wound evaluation, including the calculation of wound area. Thus, wound size and depth are the most common data points collected by wound assessment tools.1 Other essential aspects of a complete wound evaluation include documentation of the quality and appearance of the wound bed, edge, and periwound tissue; observation of the color and quantity of exudate or drainage; and assessment of circulation, bioburden, and infection risk.1,2 By adopting such a comprehensive approach, health care practitioners can enhance the accuracy of wound diagnosis and assessment, leading to more informed and effective decisions in the management of wounds.
The WCCC TWG is a volunteer, interdisciplinary group of clinicians, researchers, health systems, government agencies, payers, manufacturers, and patients who recognize the crucial need for reliable wound assessment tools and devices that support effective wound management across the continuum of care and meaningful clinical research end points. Therefore, the WCCC TWG has made the identification and reporting of devices that assist wound assessment an immediate priority.
PAR is a measure of wound healing that quantifies the reduction in wound surface area over time. Established over 2 decades ago by Sheehan et al,3 PAR in diabetic foot ulcer area after 4 weeks of observation is a robust predictor of healing at 12 weeks. Additionally, a recent publication of a post hoc analysis of a large multicenter randomized clinical trial reported findings to support the use of 4-week PAR as a prognostic indicator for diabetic foot ulcers consistent with previously published studies.4 This coincides with the most often reported PAR analysis time frames amongst diabetic foot ulcer and venous leg ulcer studies.5,6 PAR was also confirmed as a surrogate end point in a recent review that included a description of the PAR measurement methods used in 17 clinical trials; these methods were photographs and traced planimetry (2 trials) or photographs and computer-based planimetry (6 trials), manual tracing planimetry (2 trials), computer-based planimetry (4 trials), and length and width measurements (3 trials).6
Another measure of wound healing, PVR, incorporates changes in wound depth and volume in its quantification. Wound volume may be obtained in various ways, such as ruler measurements, liquid volumetric fills, or 3D techniques.7 There is less evidence published about wound PVR than about PAR, perhaps due to the complexities of obtaining volumetric measures.
Monitoring wound PAR and PVR over the course of treatment can help clinicians identify when a therapy has been efficacious in directing the wound into a healing trajectory or in identifying stalled or deteriorating wounds, thus alerting the clinician that a change in treatment may be necessary. Consequently, PAR and PVR are pivotal end points that provide a precise, quantitative foundation for evaluating wound healing trends, enabling health care providers to make informed decisions about treatment modalities and strategies.
A mission of the WCCC TWG is to support the use of PAR and/or PVR in clinical trials, where PAR and PVR serve as primary end points that are accepted by the FDA.8–10 The Wound-care Experts/FDA-Clinical Endpoint Project (WEF-CEP), which is supported by the Association for the Advancement of Wound Care and the Wound Healing Society, has published evidence to support use and adoption of these primary end points in clinical trials.8–10 Moreover, leveraging imaging tools that determine PAR and/or PVR helps clinicians track these important metrics and supports better patient outcomes. To support these primary end points, the WCCC TWG has been specifically focused on identifying and reporting the reliability of wound imaging devices capable of measuring PAR and/or PVR as their primary function or in conjunction with other wound parameters.11,12
The WCCC TWG conducted the present study to identify and describe the various features associated with wound imaging devices. The aim was to establish the necessary features of a device that would allow clinicians to have faith in the fidelity of the information documented with imaging devices used by various providers. As part of the study, a questionnaire was developed to survey manufacturers regarding their imaging devices’ publicly available information, seeking confirmation and further technical information to help inform the clinician or researcher using PAR or PVR to guide their decision-making.
Methods
The WCCC TWG collected initial data on current devices used in wound care to image and measure wounds. This information was then used to develop a list of manufacturers and devices to investigate further by collecting specific details regarding the devices’ measurement capabilities. From these data, a working spreadsheet of device features was generated. Members of the WCCC TWG were then selected to participate in a TWG PAR subcommittee to develop a clinical relevance and capabilities questionnaire to describe specific wound imaging device features deemed important for the reliability and validity of PAR and PVR measurement. The relevant clinical areas of interest included the device’s ability to measure length, width, and depth, and to calculate PAR and/or PVR; the trustworthiness of the measurement to inform clinical decision-making; wound tissue character and assessment abilities; photo editing and adjustment capability; photo review and PAR and/or PVR tracking; and convenience or usability.
It is important to note that medical devices intended for use on human subjects, such as wound imaging tools, are categorized into 1 of 3 risk-based classes by the FDA. Additionally, those devices marketed in the United States must be listed on the FDA’s Establishment Registration & Device Listing database regardless of class designation.13 The FDA Medical Devices Product Classification database provides information to the public about types of medical devices and their classifications, which are regulated by the Center for Medical Devices and Radiological Health (CDRH).14 Medical devices listed under product code FXN (Tape, Camera, Surgical) are Class 1 medical devices considered low risk and exempt from 510(k) premarket notification of substantial equivalence according to the Federal Food, Drug, and Cosmetic Act (21 CFR 878.4160).15 Medical devices with unique device identifiers may also be listed in the GUDID, which can be searched on AccessGUDID, another public database.16
Data were procured via searches of these publicly available FDA databases, journal articles, and manufacturers’ websites, as well as from WCCC TWG members’ first-hand clinical wound management experiences. Initially, FDA-registered establishments were cross-referenced with the FDA product code database and the GUDID for study inclusion. Additional inclusion criteria were devices currently marketed in the United States that are capable of length and width wound measurement functions and are from FDA-listed establishments with the product code FXN. Additional exclusion criteria included devices that do not measure wound length and width, investigational devices, repackaged and/or relabeled devices, and discontinued devices. The PAR subcommittee performed several rounds of data acquisition for the identified wound imaging devices that met all inclusion criteria. Subsequently, several phases of outreach to representatives of the listed establishments with wound imaging devices in the United States were undertaken in 2022, from October 2023 through January 2024, and lastly, April 2024 through June 2024 for feedback on the findings.
Results
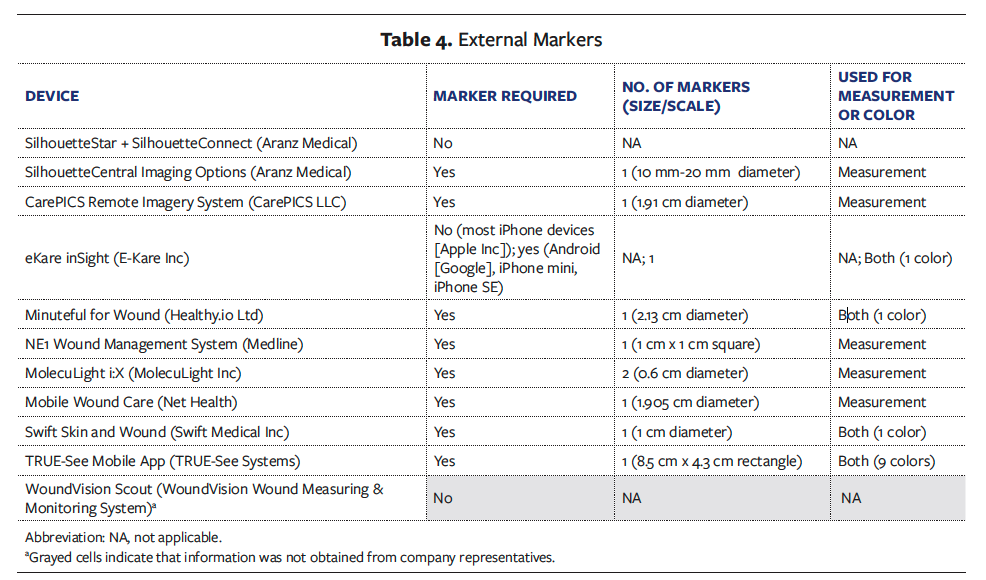
Thirteen FDA-registered establishments with 14 wound imaging devices classified under product code FXN met the inclusion and exclusion criteria. Among them, representatives from 10 companies, accounting for 77%, submitted their completed questionnaires (Table 1). Reasons for nonparticipation included the subcommittee’s inability to obtain a response from a qualified individual at the establishment or concerns about proprietary information or marketing disadvantages with participation. The following comparative summaries and tables of information were compiled based on the data acquired concerning 11 of the 14 potential wound imaging devices.
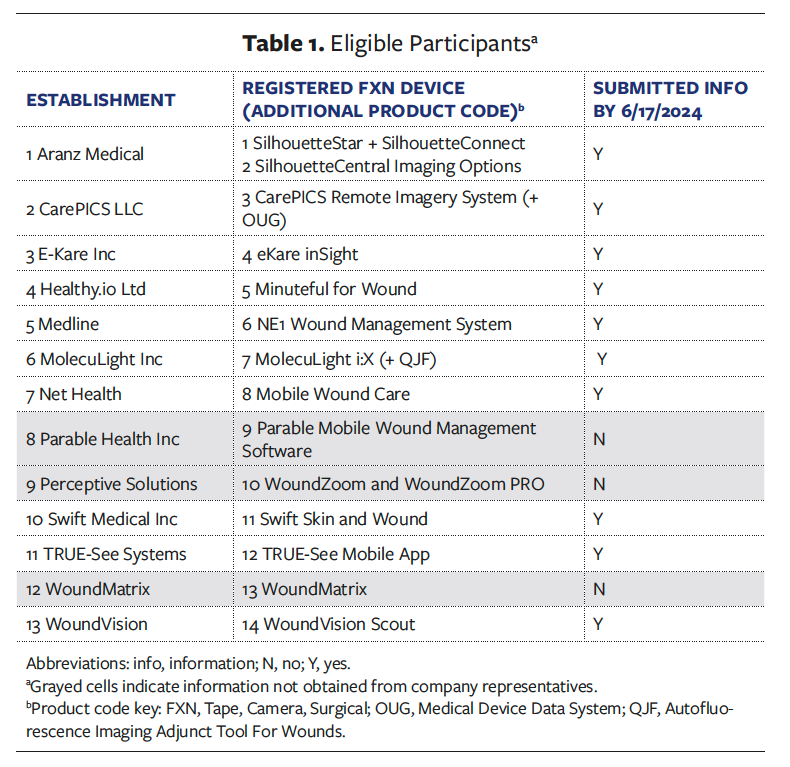
Automatic measures of length, width, depth, and calculations of PAR and/or PVR
Table 2 summarizes the findings related to the automatic measurement abilities of the wound imaging devices. Ten devices are capable of measuring PAR automatically. Seven devices measure depth, and 3 devices automatically measure PVR. One device uses all 3 queried measurement methods (laser, 3D, and digital photography), 3 devices use 3D and digital photography methods, 1 device indicated using a 3D method only, and 5 devices use digital photography only. The measurement methods were not reported for 1 device. Area is calculated based on perimeter only by 4 devices and based on square surface area only by 1 device. All but 2 devices track the calculation methods used for each photo. This is a rapidly growing field, and several establishments indicated that they had various measurement methods or calculations under development; thus, Table 2 summarizes the capabilities of the devices as of June 2024.
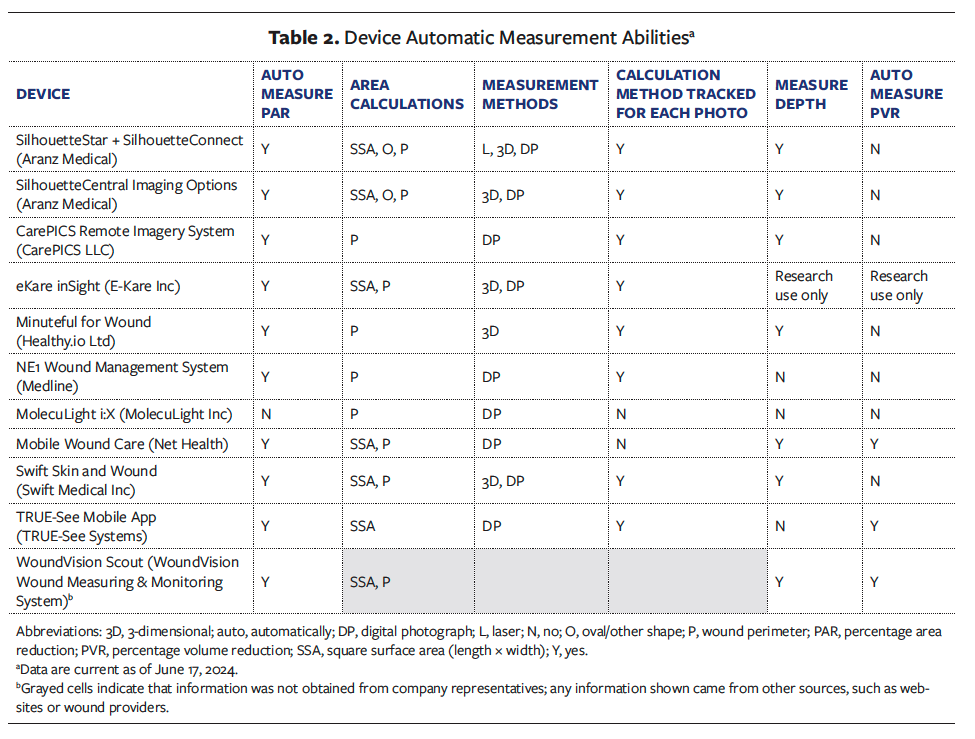
Trustworthiness of the measurement to inform clinical decision-making
Representatives reported information about measurement accuracy, reliability, and potential error sources for 7 devices. Of these, 5 devices included accessible published literature further supporting their findings. Table 3 highlights the reported accuracy values to 5% increments due to the proprietary nature and variety of internal testing methods supplied.
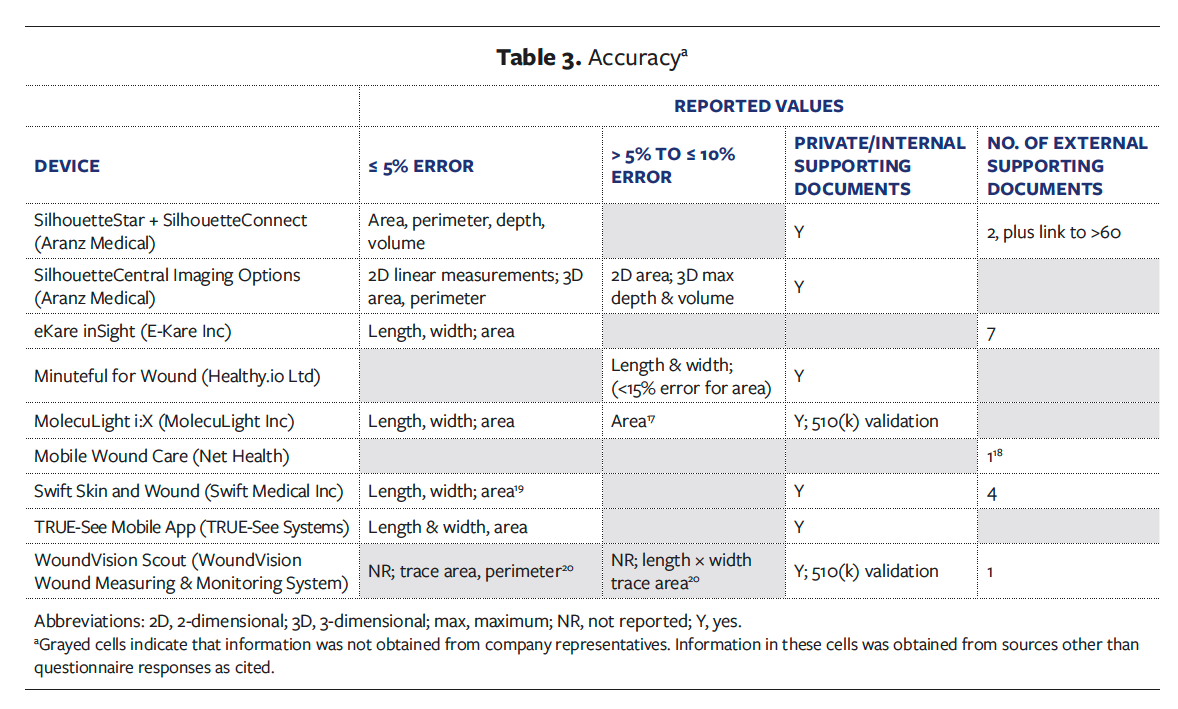
Table 4 summarizes the extent to which each wound imaging device utilizes external markers. Six devices use circular reference markers with scales of approximately 1 cm to 2 cm in diameter, 1 device uses a 1 cm × 1 cm square reference marker, and another device utilizes a larger rectangular scale of 8.5 cm × 4.3 cm. One device also has a marker-based option for devices that are not compatible with the markerless option (Table 4).
Some devices incorporate other features to ensure image quality, such as the ability to detect and adjust focus (7 devices), offering tutorials or other assistance or guidance to help the user with distance or angle (9 devices), or providing an overlay of the most current photo (9 devices) for consistency of images. Concerning the issue of image distortion, 6 devices offer solutions for identifying and correcting skew (Table 5).
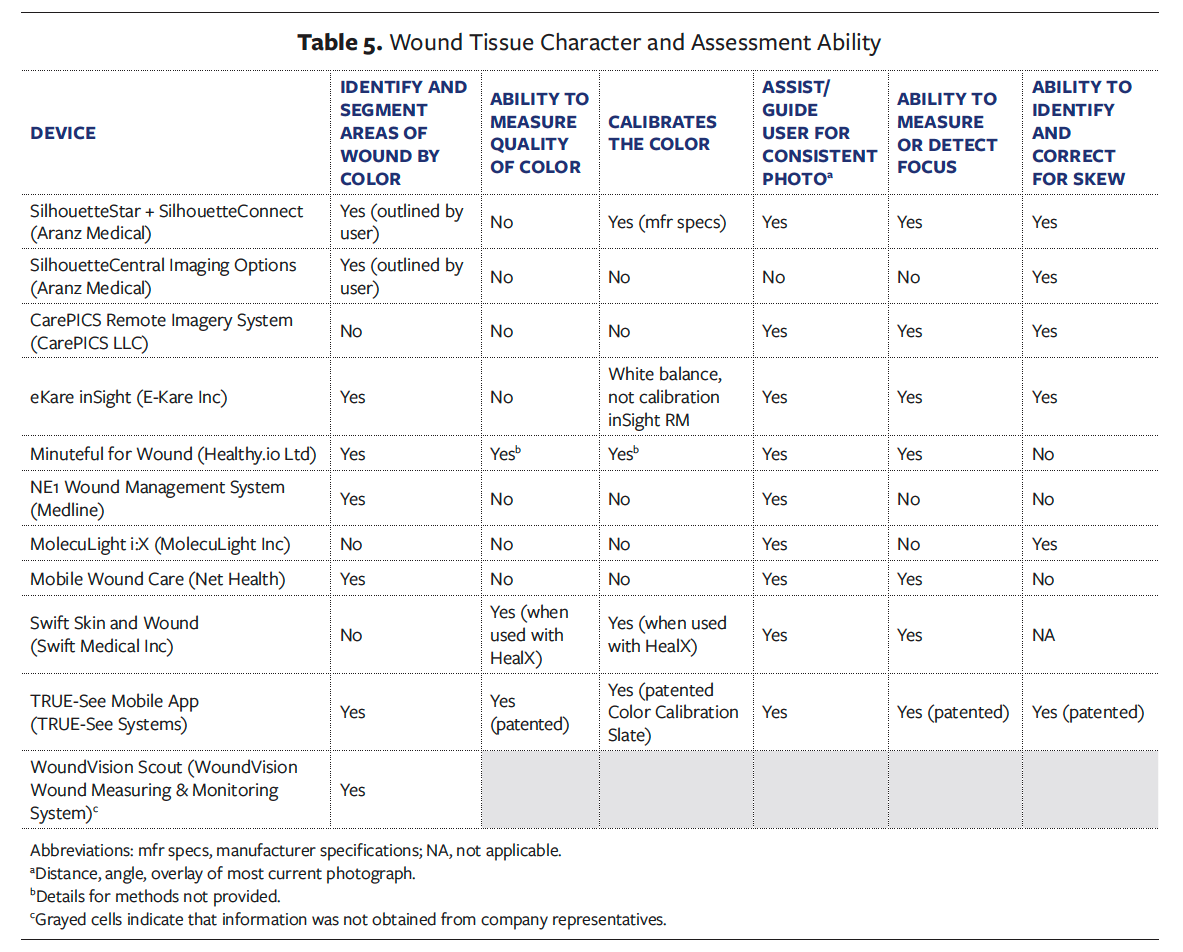
Most devices offer valuable features that allow the clinician to adjust the wound measurement area or border and reveal to the user where the wound was previously measured. This allows for comparison between serial wound images to track area calculations over time. Additionally, 4 devices were reported to maintain an audit log that documents any changes made to measurements for full transparency (Table 6).
Wound tissue character and assessment abilities
Eight devices require the use of an external marker or markers adjacent to the wound for either measurement or color calibration adjustments (Table 4). Eight devices were reported to have the capability to identify and segment wound tissue by color, 3 reportedly measure the quality of the color, and 4 reportedly calibrate the color. However, only 1 imaging device provided supporting documentation and has issued patents related to color quality and calibration (Table 5).21,22
Photo integrity
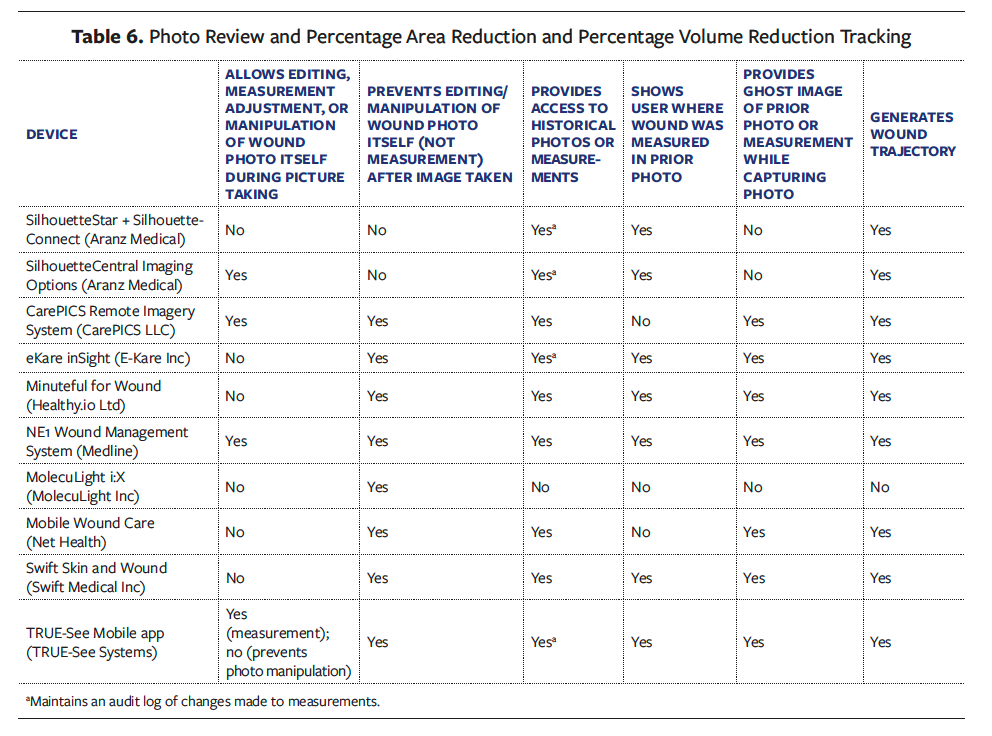
Three devices allow the clinician to edit, adjust measurement, or manipulate the wound photo during the picture-taking process before finalizing it for documentation, and 8 devices prevent editing or manipulation of the wound photo itself (not measurement) after the image has been taken (Table 6).
Photo review and PAR and/or PVR tracking
Clinicians and researchers rely on wound image consistency to effectively compare and evaluate PAR and PVR over time. Photo consistency improves with device features that (1) provide access to historical photos or measurements (reported by 9 devices), (2) show the user where the wound was measured in a prior photo (provided by 7 devices), or (3) provide a ghost image of the prior photo or measurement while capturing a new image (available in 7 devices). Additionally, 9 devices have the capability to track and generate wound trajectory based on the PAR or PVR measurement end point (Table 6).
Convenience and usability
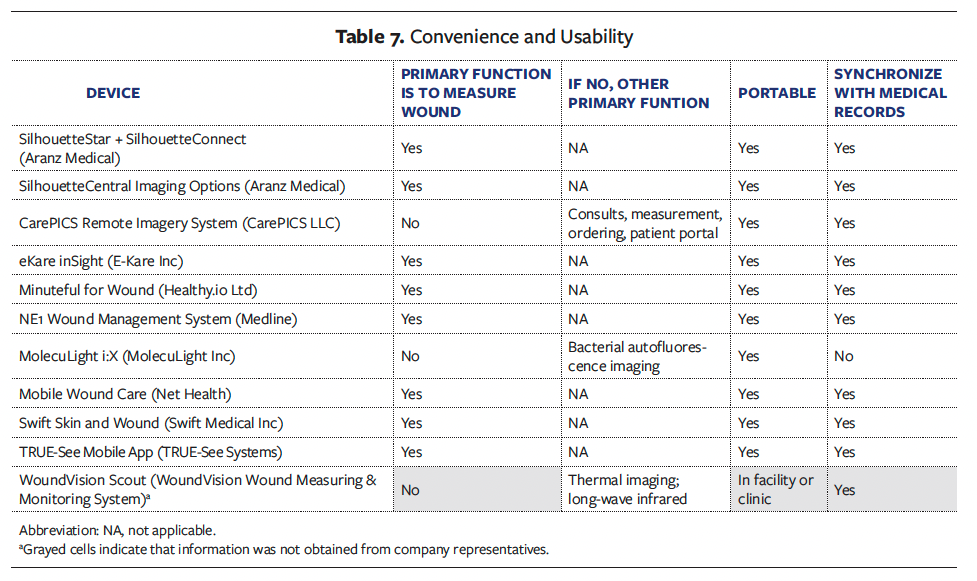
Eight devices obtain wound measurements as their primary function, and 3 devices can measure the wound as a secondary capability. All the devices are portable, allowing them to be easily incorporated into the wound assessment procedures for various settings. More importantly, 10 of the 11 devices confirmed that they can synchronize with electronic medical records systems, to ensure documentation of the wound images within the patient medical records (Table 7).
Discussion
The measurement of PAR is a meaningful end point for monitoring the trajectory of wound healing in clinical practice and research because it provides early indications of the effectiveness of treatment.4 Devices that can be used early to accurately measure PAR and/or PVR, especially in large, irregular wounds with depth, can assist the recognition of healing responses before visual changes are physiologically apparent. Innovations in digital imaging are an increasingly important component of wound assessment because 2D and 3D measurements can address many of the limitations associated with simple ruler measurements of length and width for determining wound surface area.23–25 Wound images that are reliable, repeatable for comparison, and secured from manipulation after being obtained improve the validity of PAR and PVR. Devices that can reliably capture wound images and automatically measure PAR improve upon standard manual ruler measurements of wounds, which require direct tissue contact and have been shown to inaccurately overestimate wound area by up to 40%.7,26
Digital wound imaging devices are more efficient and accurate than simple ruler measurements23,25,27; however, their device-dependent measurement and modeling algorithms can affect reliability and validity. For example, area measurements may be calculated based on wound perimeter, or by using various shape-
related equations, or by various triangulation or modeling methods. Regardless of the method, determining the wound edge for any of the device or manual measurement techniques can be challenging. Furthermore, measurement devices with 3D imaging have been shown to be more accurate than planimetric measurements by devices that utilize 2D imaging based on flat, linear views.28,29
Devices capable of automatic PAR measurements provide valuable clinically relevant and validated information for decisions regarding progression of the wound repair process and assist clinicians in decision-making for treatment.8,9 Wound measurements are complicated by irregular wound shapes, indistinct wound edges, uneven depths, skin color, and human error processes related to positioning, lighting, training, and computational issues, for example.30 Unique factors that can affect the accuracy of device-
related wound measurements include the reference scale used, the device’s ability to adjust for skew, and color calibration abilities to reduce color distortion for improved human skin assessment beyond the capability of the naked eye.31–33
Standards for acceptable levels of wound measurement variability, including scale and skew, have not been established for any measurement methods. Given the clinical and billing implications of accurate measurements, it is imperative that the method and accuracy of the electronic measurements are clear to the clinician. Originating in cartography, the basic science of measuring an unknown object in relationship to a known object is well established; the larger the scale, the more accurate the measurement.34 Likewise, skew detection and correction are important features; introducing skew in a photo has been shown to produce a 34.8% error in wound area measurement.35
The problem of introducing error when converting a piece of continuous information (eg, real-life wound size) to a discrete representation (eg, individual pixels representing the wound on an image) is called quantization error.36 This error is dependent on the detail of an object in the photo, or its spatial resolution.37 In short, the greater the detail of the scale in the photo, the more accurate the measurements. For example, if a software program such as OpenCV identified a known circle with a diameter of 2 cm in a photo, and counted 100 pixels across that circle, the scale would be 2 cm equals 100 pixels, or 1 cm equals 50 pixels. Just as on a map, the scale is then used to extrapolate the size of the unknown object. If the unknown object is 2 cm, then the ratio of the object to the scale is 1 to 1 (1:1), introducing little margin of error. However, if an object is 5 times the size of the scale, then the ratio is 5 to 1 (5:1), which introduces a greater margin of error. Thus, the larger the scale, the more accurate the extrapolated measurement. If no scale is present in the photo, it is important that the clinician understand how the measurements are accurately derived.
Wound convexity, concavity, and circumferentiality add further challenges to the reliability of all measurement techniques. Wound depth measurements have an increased potential for variability when using either manual or imaging devices due to the inherent nature of an uneven wound base upon which clinicians attempt to measure the deepest part of the wound.25,38,39 Despite all these factors, several wound measurement tools and techniques have been reported to fall within 11% variance.23–25,40 Regardless of the tools used, wound measurement techniques for PAR and/or PVR have been found to be valuable and clinically meaningful end points for research and clinical practice.4,6,8,9
It is important for clinicians to be mindful of best practices when using devices to measure PAR and/or PVR, such as consistent positioning and lighting, which can affect wound dimensions and appearance, thus affecting PAR measurements and more so affecting depth and PVR measurements.25,35,41 Therefore, following manufacturer guidelines and best practices for digital wound measurements is important to improve accuracy of repeated measurements for monitoring change over time.25,30,41 Likewise, while slight variations in length, width (up to 5%), and area (up to 10%) may occur with device calculations, including methods to correct for skew, using sophisticated 3D modeling and/or detection based on reference markers significantly enhances a device’s overall wound measurement trustworthiness.
The device manufacturers that engaged with this survey research demonstrated a willingness to share information that helped in identifying a list of important capabilities to enhance the trustworthiness of device-generated wound measurements, including PAR and PVR. This serves as a foundation to build on for the transparent development of measurement standards that can be readily used by manufacturers to enhance wound care delivery and research, as well as new device, drug, or biologic approvals for market in wound care. Devices that automatically calculate wound length, width, and PAR decrease human measurement error and perform more consistently across various providers than other techniques.20
Limitations
Given the broad availability of mobile phone cameras, a limitation of this study is that imaging tools are available that may be used to capture wound photos and measurements but are not registered with the FDA for various reasons, such as being mobile applications instead of medical devices. The inclusion and exclusion criteria focused specifically on medical devices with the FXN (Tape, Camera, Surgical) product code for the eligible population to establish a baseline functionality in an expansive, searchable database. This eliminated bias that would be impractical to overcome if all potential imaging applications, even those not intended for use in a medical setting, were considered. The authors of the present study acknowledge that the initial investment in new imaging technologies capable of PAR and/or PVR calculations can be cost prohibitive for some researchers or facilities. This includes not only the purchase price but also ongoing maintenance and operational costs, which can limit overall access to these devices.
Another limitation of the present study was not achieving full participation from some manufacturers despite multiple outreach attempts, with 1 manufacturer citing the lack of a uniform standard methodology for determining the accuracy of wound imaging measurement processes. The survey identified key opportunities to improve the field through adopting proven methods for measurement and color accuracy along with uniform standards, such as the ASTM International E177-20, Standard Practice for Use of the Terms Precision and Bias in ASTM Test Methods.42 Although the questionnaire developed for and used in the present study identified important features to assist in selecting devices for clinicians and researchers, and for new product approvals, there were limitations when evaluating the technical aspects of measurement capture and processing methods. There is variation in the approaches used by manufacturers for determining the measurement error or reliability, and there is a lack of consistent testing methods across the industry that makes it difficult to assess a particular device against another. However, opportunity exists to establish standards in the future.
Conclusion
The goal of the present WCCC TWG research was 2-fold. First, the WCCC sought to identify currently available FDA-listed devices for the measurement of PAR and/or PVR to support their use as primary end points. Secondly, the WCCC sought to identify features of measurement devices that support their reliability and the validity of the wound images used to calculate PAR, PVR, and wound healing trajectory, and to aid in clinical decision-making. In the absence of recognized standards for review or validation of device features related to image quality, integrity, consistency, reliability, and security for PAR and PVR measurement, developers and clinicians can refer to the capabilities of current devices identified in the present survey that support the use of these devices in wound care practice. Moreover, the results of this investigation support the use of advanced imaging technologies to enhance the overall accuracy and reliability of wound measurements, thus supporting the collection of PAR and PVR data as an indicator of wound trajectory. The authors of the present study encourage the development of measurement standards for wound imaging devices that developers can use for instrument reliability and validity testing.
Author and Public Information
Authors: Holly Korzendorfer, PhD, MPT, CWS1,2; Peggy Dotson, RN, BS1; Francis James, BFA, SOC1,3; Windy Cole, DPM, CWSP1,4; and Alisha Oropallo, MD1,5-7
Affiliations: 1Tools Work Group, Wound Care Collaborative Community (WCCC); 2Marist University, Poughkeepsie, NY, USA; 3TRUE-See Systems, New Orleans, LA, USA; 4Kent State University College of Podiatric Medicine, Independence, OH, USA; 5Director, Department of Vascular and Endovascular Surgery, Northwell Health, Lake Success, NY, USA; 6Professor, Zucker School of Medicine, Hofstra University/Northwell Health; 7Professor, Feinstein Institutes of Medical Research, Northwell Health
Acknowledgments: The authors would like to express their gratitude to all the companies that took the time to contribute information for the completion of this manuscript, including Aranz Medical, CarePICS LLC, E-Kare Inc, Healthy.io Ltd, Medline, MolecuLight Inc, Net Health, Swift Medical Inc, TRUE-See Systems, and WoundVision.
Disclosure: Francis James is the founder and chief product officer for TRUE-See Systems. The other authors disclose no financial or other conflicts of interest.
Ethical Approval: This study did not involve human subjects research and was therefore not subject to institutional review board approval.
Correspondence: Holly Korzendorfer, PhD; Marist University, 3399 North Rd, AH-215, Poughkeepsie, NY 12601; Holly.franzen-korzendorfer@marist.edu
Manuscript Accepted: March 25, 2025
Recommended Citation
Korzendorfer H, Dotson P, James F, Cole W, Oropallo A. An overview and survey of US Food and Drug Administration–registered wound imaging devices capable of determining percentage area reduction and/or percentage volume reduction. Wounds. 2025;37(5):210-219. doi:10.25270/wnds/24201
References
1. Smet S, Probst S, Holloway S, Fourie A, Beele H, Beeckman D. The measurement properties of assessment tools for chronic wounds: a systematic review. Int J Nurs Stud. 2021;121:103998. doi:10.1016/j.ijnurstu.2021.103998
2. Gray D, White R, Cooper P, Kingsley A. Applied wound management and using the wound healing continuum in practice: technical guide. 2010;5:131-139. https://www.semanticscholar.org/paper/Technical-Guide-APPLIED-WOUND-MANAGEMENT-AND-USING-Gray-White/21b86ad2d6a6d9c057c5aba528b12914867ec40e
3. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26(6):1879-1882.
4. Serena T, Yaakov S, Yaakov R, King E, Driver VR. Percentage area reduction at week 4 as a prognostic indicator of complete healing in patients treated with standard of care: a post hoc analysis. J Wound Care. 2024;33(Sup9):S36-S42. doi:10.12968/jowc.2024.0141
5. Gwilym BL, Mazumdar E, Naik G, Tolley T, Harding K, Bosanquet DC. Initial reduction in ulcer size as a prognostic indicator for complete wound healing: a systematic review of diabetic foot and venous leg ulcers. Adv Wound Care. 2023;12(6):327-338. doi:10.1089/wound.2021.0203
6. Lammert A, Kiehlmeier S, Dissemond J, Munter KC, Schnorpfeil W, Pohl J. Percentage area reduction as surrogate for complete healing of hard-to-heal wounds: a review of clinical trials. J Wound Care. 2024;33(10):737-755. doi:10.12968/jowc.2023.0117
7. Jørgensen LB, Sørensen JA, Jemec GB, Yderstræde KB. Methods to assess area and volume of wounds – a systematic review. Int Wound J. 2015;13(4):540-553. doi:10.1111/iwj.12472
8. Driver VR, Gould LJ, Dotson P, et al. Identification and content validation of wound therapy clinical endpoints relevant to clinical practice and patient values for FDA approval. Part 1. Survey of the wound care community. Wound Repair Regen. 2017;25(3):454-465. doi:10.1111/wrr.12533
9. Driver VR, Gould LJ, Dotson P, Allen LL, Carter MJ, Bolton LL. Evidence supporting wound care end points relevant to clinical practice and patients’ lives. Part 2. Literature survey. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2019;27(1):80-89. doi:10.1111/wrr.12676
10. Gould LJ, Liu J, Wan R, Carter MJ, Dotson MP, Driver VR. Evidence supporting wound care end points relevant to clinical practice and patients’ lives. Part 3: The Patient Survey. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2021;29(1):60-69. doi:10.1111/wrr.12872
11. Rennie M, Dotson P. Wound assessments to measure endpoints: an update from the Wound Care Collaborative Community (WCCC). Wounds. 2023;35(9):8-9. doi:10.25270/wnds/350923-2
12. Oropallo A, Dotson P, Brindle T, Driver VR, Gould L. Need for percent area reduction and percent volume reduction measurements in diagnostic wound imaging: a statement from the Wound Care Collaborative Community—part 1. Wounds. 2024;36(2). Accessed June 17, 2024. https://www.hmpgloballearningnetwork.com/site/wounds/innovations-research-methods-and-reporting/need-percent-area-reduction-and-percent
13. Establishment Registration & Device Listing. Accessed June 17, 2024. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfrl/textsearch.cfm
14. Product Classification. Accessed June 17, 2024. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPCD/classification.cfm
15. Product Classification. Accessed June 17, 2024. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?id=FXN
16. AccessGUDID - Identify Your Medical Device. Accessed June 17, 2024. https://accessgudid.nlm.nih.gov/
17. Chan KS, Lo ZJ. Wound assessment, imaging and monitoring systems in diabetic foot ulcers: a systematic review. Int Wound J. 2020;17(6):1909-1923. doi:10.1111/iwj.13481
18. Budman J, Keenahan K, Acharya S, Brat GA. Design of a smartphone application for automated wound measurements for home care. Iproceedings. 2015;1(1):e16. doi:10.2196/iproc.4703
19. Wang SC, Anderson JAE, Evans R, et al. Point-of-care wound visioning technology: reproducibility and accuracy of a wound measurement app. Facchiano A, ed. PLoS One. 2017;12(8):e0183139. doi:10.1371/journal.pone.0183139
20. Langemo D, Spahn J, Spahn T, Pinnamaneni VC. Comparison of standardized clinical evaluation of wounds using ruler length by width and Scout length by width measure and Scout perimeter trace. Adv Skin Wound Care. 2015;28(3):116-121. doi:10.1097/01.ASW.0000461117.90346.0d
21. James F, inventor; TRUE-See Systems LLC, assignee. System for producing consistent medical image data that is verifiably correct. US patent 10,973,412. April 13, 2021.
22. James F, Pattam S, inventors; TRUE-See Systems LLC, assignee. System for producing three-dimensional medical images using a calibration slate. US patent 11,961,260. April 16, 2024.
23. Lucas Y, Niri R, Treuillet S, Douzi H, Castaneda B. Wound size imaging: ready for smart assessment and monitoring. Adv Wound Care. 2021;10(11):641-661. doi:10.1089/wound.2018.0937
24. Shah A, Wollak C, Shah JB. Wound measurement techniques: comparing the use of ruler method, 2D imaging and 3D scanner. J Am Coll Clin Wound Spec. 2015;5(3):52-57. doi:10.1016/j.jccw.2015.02.001
25. Wendland DM, Taylor DWM. Wound measurement tools and techniques: a review. J Acute Care Phys Ther. 2017;8(2):42-57. doi:10.1097/JAT.0000000000000050
26. Rogers LC, Bevilacqua NJ, Armstrong DG, Andros G. Digital planimetry results in more accurate wound measurements: a comparison to standard ruler measurements. J Diabetes Sci Technol. 2010;4(4):799-802. doi:10.1177/193229681000400405
27. Sprigle S, Nemeth M, Gajjala A. Iterative design and testing of a hand-held, non-contact wound measurement device. J Tissue Viability. 2012;21(1):17-26. doi:10.1016/j.jtv.2011.09.001
28. Liu C, Fan X, Guo Z, Mo Z, Chang EIC, Xu Y. Wound area measurement with 3D transformation and smartphone images. BMC Bioinformatics. 2019;20(1):724. doi:10.1186/s12859-019-3308-1
29. Ayaz I, Shaheen E, Aly M, et al. Accuracy and reliability of 2-dimensional photography versus 3-dimensional soft tissue imaging. Imaging Sci Dent. 2020;50(1):15-22. doi:10.5624/isd.2020.50.1.15
30. European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline. Emily Haesler (Ed.). EPUAP/NPIAP/PPPIA; 2019. Accessed June 23, 2024. https://internationalguideline.com/2019
31. Penczek J, Boynton PA, Splett JD. Color error in the digital camera image capture process. J Digit Imaging. 2014;27(2):182-191. doi:10.1007/s10278-013-9644-1
32. Amani M, Falk H, Jensen OD, Vartdal G, Aune A, Lindseth F. Color calibration on human skin images. In: Tzovaras D, Giakoumis D, Vincze M, Argyros A, eds. Computer Vision Systems. Springer International Publishing; 2019:211-223. doi:10.1007/978-3-030-34995-0_20
33. Marguier J, Bhatti N, Baker H, Harville M, Süsstrunk S. Assessing human skin color from uncalibrated images. Int J Imaging Syst Technol. 2007;17(3):143-151. doi:10.1002/ima.20114
34. Yao X. Georeferencing, Geocoding. In: Kitchin R, Thrift N, eds. International Encyclopedia of Human Geography. Elsevier; 2009:458-465. doi:10.1016/B978-008044910-4.00448-X
35. Rennert R, Golinko M, Kaplan D, Flattau A, Brem H. Standardization of wound photography using the wound electronic medical record. Adv Skin Wound Care. 2009;22(1):32. doi:10.1097/01.ASW.0000343718.30567.cb
36. Claff B. Quantization Error in Practice. September 19, 2015. Accessed December 3, 2024. https://www.photonstophotos.net/GeneralTopics/Sensors_&_Raw/Quantization_Error_in_Practice.htm
37. Shannon CE. Communication in the presence of noise. Proc IRE. 1949;37(1):10-21. doi:10.1109/JRPROC.1949.232969
38. Constantine RS, Bills JD, Lavery LA, Davis KE. Validation of a laser-assisted wound measurement device in a wound healing model. Int Wound J. 2016;13(5):614-618. doi:10.1111/iwj.12328
39. Flanagan M. Wound measurement: can it help us to monitor progression to healing? J Wound Care. 2003;12(5):189-194. doi:10.12968/jowc.2003.12.5.26493
40. A clinimetric analysis of wound measurement tools. Accessed July 17, 2024. http://www.worldwidewounds.com/2006/january/Fette/Clinimetric-Analysis-Wound-Measurement-Tools.html
41. Estocado N, Black J. Ten top tips: wound photo documentation. Wounds Int. 2019;10(3):8-12.
42. American Society for Testing and Materials. ASTM E177-20. Stand Pract Use Terms Precis Bias ASTM Test Methods. 2020;ASTM International. doi:10.1520/E0177-20


















