Obstructive Sleep Apnea is an Independent Risk Factor for Split-Thickness Skin Graft Failure
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. Obstructive sleep apnea (OSA) is an underdiagnosed and undertreated disease that has been significantly associated with diabetes, cardiovascular disease, peripheral arterial disease, and poor wound healing. Objective. To determine whether or not OSA is an independent risk factor for split-thickness skin graft (STSG) failure in patients with chronic lower extremity (LE) wounds, given OSA’s disease burden to common comorbidities in the LE wound population. Methods. A retrospective chart review of chronic LE wounds managed with STSG between December 2014 and December 2022 was conducted. Patients with a diagnosis of OSA (“OSA”) were compared with patients without an OSA diagnosis (“Non-OSA”). Results. Overall, 14.9% of patients had OSA (n = 25) and 85.1% (n = 143) did not have OSA. Patients with OSA had a significantly higher median body mass index than the Non-OSA group (32.9 and 28.2, respectively; P = .013) and a higher rate of chronic obstructive pulmonary disease (16.0% and 4.2%, respectively; P = .043). Compared with patients without OSA, patients with OSA had more superficial wounds (P = .027) and received thinner skin grafts (P = .016). Compared with the Non-OSA group, wounds in the OSA group had significantly higher rates of graft failure (OSA 30.6% vs Non-OSA 15.9%; P = .034), infection (OSA 27.8% vs Non-OSA 10.6%; P = .005), and reoperation (OSA 52.8% vs Non-OSA 31.1%; P = .010). On multivariate logistic regression, OSA remained a significant risk factor for graft failure, increasing the odds of graft failure by 8.6 times (P = .040). Conclusion. OSA is an independent predictor of graft failure in a highly comorbid population with chronic LE wounds. Preoperative identification of these high-risk patients should be undertaken by anesthesia, sleep medicine, and surgical teams to improve outcomes.
Chronic wounds represent a substantial clinical and global health care burden.1 Diabetes mellitus (DM), a prominent contributor to chronic lower extremity (LE) ulceration, affects an estimated half billion individuals worldwide, and the number is projected to increase by 25% to 51% by 2045.2 Established comorbidities such as poorly controlled DM, chronic kidney disease (CKD), peripheral arterial disease (PAD), and aging are acknowledged impediments to wound healing3 because they compromise tissue oxygenation, vascular integrity, and the effective delivery of immune mediators to wounds.4,5
Complicating this landscape is obstructive sleep apnea (OSA), a condition affecting nearly 1 billion people worldwide, yet only diagnosed in 20% of the population.6-8 OSA, which is characterized by repetitive partial or total upper airway collapse leading to sleep fragmentation and intermittent oxygen desaturation, is prevalent yet often severely under-recognized, both among patients with the condition and by physicians who do not specialize in its management.3-5,9
Multiple studies have demonstrated that there is an association between OSA and delayed wound healing10-13; however, none have evaluated the effect of OSA on the outcomes of split-thickness skin graft (STSG), a common first-line procedure for chronic wound closure, nor on any other reconstructive procedures. Similar to the requirements for successful wound healing by primary or secondary intention, a successful graft requires adequate oxygenation and nutrient supply.10-15 There persists a gap in the current literature evaluating the effect of OSA on STSG outcomes for the chronic LE wound population. Thus, the present study aims to investigate the role of OSA in STSG outcomes, with the goal of refining patient care strategies and improving outcomes in reconstructive plastic surgery and wound healing
Methods
Patient population and variables collected
Following Medstar-Georgetown institutional review board (STUDY00004145) approval, a retrospective chart review of patients undergoing STSG for chronic LE wounds between December 2014 and December 2022 was conducted. Patients were included if they were seen in the wound clinic for a chronic nonhealing LE wound (ie, a wound present for ≥3 months that did not heal with conservative treatment). Wound etiologies included nonhealing wounds due to diabetes, ischemia, venous stasis, pressure, autoimmune disorders, radiation, and previous traumatic injury or surgical intervention. Patients with incomplete follow-up information and wounds that were pre-treated with a synthetic dermal matrix, such as Integra (Integra LifeSciences), were excluded. Patients were included in the OSA group if they had clinical chart documentation of an active OSA diagnosis by a primary care provider or sleep medicine specialist.
Electronic medical records (EMRs) were reviewed for patient demographics, wound characteristics, anesthesia characteristics, surgical details, and postoperative outcomes. Demographic data included sex, age, body mass index (BMI), and comorbid conditions, including a diagnosed history of OSA. The Charlson Comorbidity Index was used to calculate each patient’s overall comorbidity burden.16 American Society of Anesthesiologists (ASA) Physical Status Classification and Mallampati scores were collected. The Mallampati score is a classification system that predicts intubation difficulty and is associated with airway obstruction risk in OSA.17 Wound characteristics, as defined on the date of surgery, included wound length, width, total surface area, depth, and location. Surgical details included STSG thickness, postoperative dressing type, and wound bed microbiology results. STSG thickness was classified into 4 groups: very thin (<0.15 mm), thin (0.15 mm-0.3 mm), moderate (0.31 mm-0.45 mm), and thick (0.46 mm-0.6 mm).18-20 Intraoperatively, qualitative cultures were obtained via a wound swab. Incidence of positive cultures, bacteria type, polymicrobial growth, and qualitative bacterial load were recorded.
Patients were assessed at the study authors’ outpatient wound clinic on postoperative visit at day 30 (POV-30) and postoperative visit at day 60 (POV-60). The primary outcome was surgical wound healing, as measured by graft failure. Graft failure was defined as complete necrosis or removal of the graft determined by the attending surgeon in clinical notes at follow-up visits at any time during patient follow-up. Time to graft failure was calculated as the days between STSG placement and failure documentation. Secondary outcomes included infection and healing rates. Patients in the “OSA” group, who had a clinical diagnosis of OSA, were compared with patients in the “Non-OSA” group, who did not have a diagnosis of OSA.
Surgical technique and perioperative management
All patients were treated via a multidisciplinary approach by physicians within the study authors’ hospital-based wound care center, including a core group of plastic, vascular, podiatric, and orthopedic surgeons. Medical and comorbidity management was provided by a secondary team of specialists. This approach has been previously and extensively described by the senior author (C.E.A.).21-33
Prior to STSG, comorbidities were optimized by the medical team and the wound bed was prepared via serial debridement and regular dressing changes. The decision to proceed to STSG was determined by the attending surgeon according to institutional protocol, considering wound debridement cultures (when either a negative or low-growth culture was obtained). At the study authors’ institution, STSG procedures are typically performed on an outpatient basis, and patients are discharged the same day unless there is concern for medical instability, wound complexity, or social factors, in which case patients are admitted for post-
operative monitoring at the discretion of the surgical team.
On the date of STSG reconstruction, a final debridement was performed before graft inset. A Zimmer Dermatome (Zimmer Biomet) was used for STSG harvest at the anterolateral thigh. STSGs were sutured to the wound bed with 4-0 absorbable sutures. The surgical site was bolstered with a sponge. In cases of excessive edema or joint motion, negative pressure wound therapy (NPWT) was applied. All patients were strictly immobilized in a neutral position for 5 to 7 days depending on the wound location, with longer periods required for grafts placed over mobile joint surfaces such as the ankle or knee. Off-loading was achieved using total contact casts or controlled ankle motion boots when clinically appropriate.
Statistical analysis
Summary statistics are presented for the overall sample and by study groups (OSA and Non-OSA) as means (± SD), medians and IQR, and proportions (if categorical). Two sample t tests were used to examine differences in the averages of continuous variables between groups (OSA vs Non-OSA) when the normality assumption was satisfied. The Wilcoxon rank sum test was used when the normality assumption was not satisfied. The t test was used to examine differences in the averages of continuous variables between the 2 groups when the normality assumption was satisfied, and the Mann-Whitney U test was used when the normality assumption was not satisfied. Chi-square and Fisher exact tests (defined as cell counts <5) were used to investigate differences for categorical variables as appropriate. Statistical significance was defined as P < .05. Multivariate regression models were performed and included covariates that were found to be significant on univariate analysis, clinically significant comorbidities, and established predictors of postoperative outcomes to account for potential confounders. To avoid model overfitting, certain significant covariates were not included if they were not clinically relevant to the outcome. The statistical program omitted covariates that perfectly predicted failure or exhibited collinearity from the model. Results were reported as an unadjusted odds ratio (OR) with 95% CI. Stata/MP software (StataCorp LLC) was used to perform all analyses.
Results
Patient demographics and anesthesia characteristics
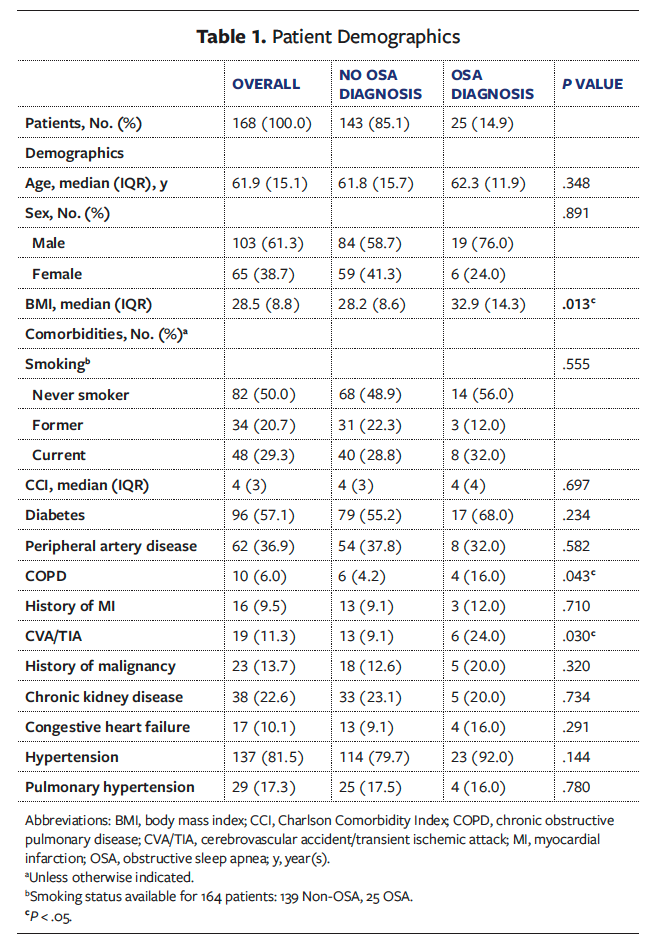
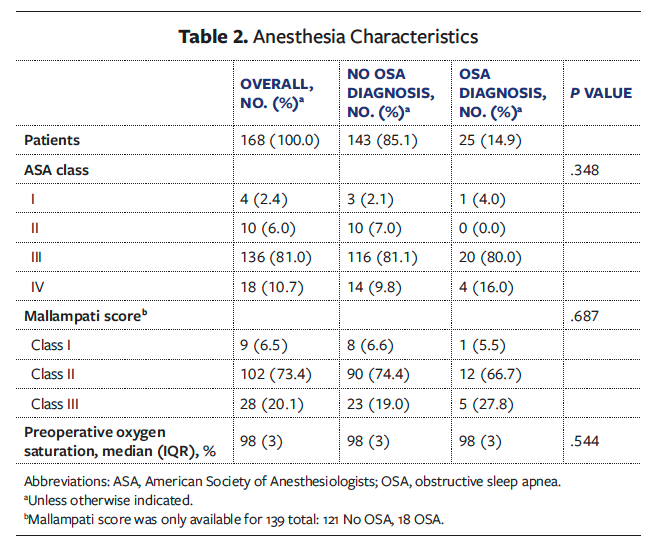
Of the 316 patient charts screened, 10 were excluded due to the absence of follow-up information and 138 were excluded due to preoperative synthetic dermal substitute use. The remaining study cohort represented 168 patients who received STSG coverage for 244 chronic LE wounds. Of these 168 patients, 25 (14.9%) had an OSA diagnosis and 143 (85.1%) did not. Patient demographics are listed in Table 1. Overall, the median (IQR) age was 61.9 (15.1) years. The majority of patients were male (n = 103 [61.3%]), and 65 patients were female (38.7%). The overall median (IQR) Charlson Comorbidity Index was 4 (3). The most common ASA class in the total cohort was class III (n = 136 [81.0%]), followed by class IV (n = 18 [10.7%]) (Table 2). Prevalent comorbidities included DM (n = 96 [57.1%]), PAD (n = 62 [36.9%]), and hypertension (n = 137 [81.5%]). Compared with patients in the Non-OSA group, patients in the OSA group had a higher median (IQR) BMI (32.9 [14.3] vs 28.2 [8.6]; P = .013) and incidence of cerebrovascular accident or transient ischemic attack (n = 6 [24.0%] vs n = 13 [9.1%]; P = .030) and chronic obstructive pulmonary disease (n = 4 [16.0%] vs n = 6 [4.2%]; P = .043). No other significant differences in demographics or comorbidities between the OSA and Non-OSA groups were observed. In the OSA cohort, 8 patients (32.0%) reported using a continuous positive airway pressure (CPAP) device at home.
Wound and surgical characteristics
Wound characteristics are summarized in Table 3. Of the 244 total wounds, 208 (85.2%) were Non-OSA wounds and 36 (14.8%) were OSA wounds. The etiology of chronic wound was similar between groups (P = .060), with diabetes (n = 94 [38.5%]) and surgical causes (n = 48 [19.7%]) being the most common. The overall median (IQR) wound surface area was 28.0 (66.0) cm2 and did not differ between groups (P = .159). However, the Non-OSA group had deeper wounds (n = 86 [41.7%] vs n = 8 [22.2%]; P = .027). Overall, most wounds were located on the lower leg (n = 78 [32.0%]), and 34.0% (n = 36) of foot wounds were located on the plantar surface. STSG thickness varied significantly between groups (P = .016), with a higher percentage of the OSA group receiving more “very thin” STSG (36.4% vs 19.9%) and a higher percentage and higher number of the Non-OSA group receiving more “intermediate” STSG (60.7% vs 33.3%).
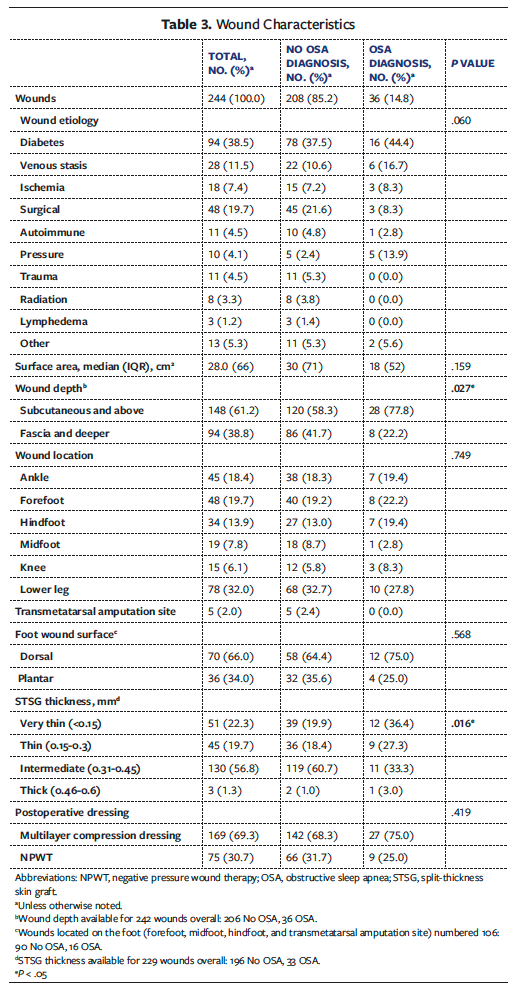
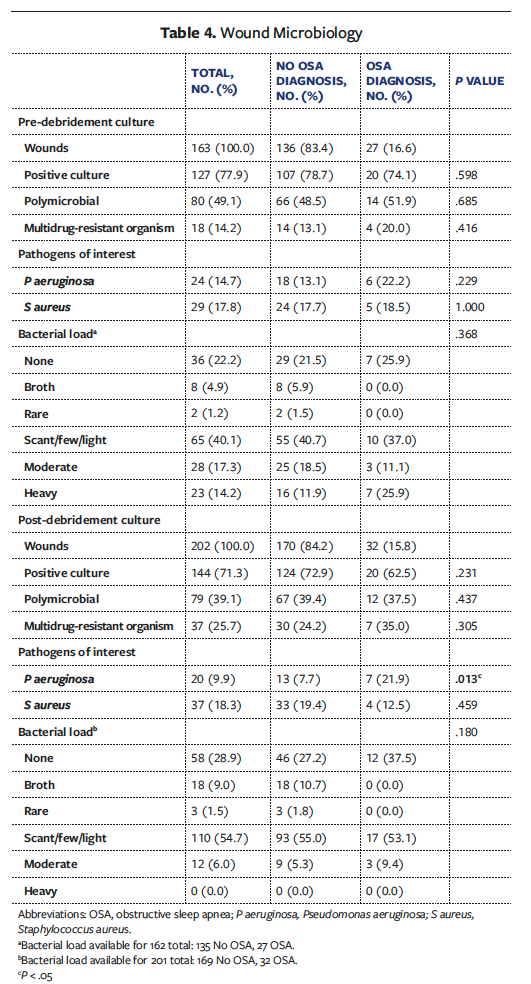
Pre-debridement cultures were available for 163 wounds overall (OSA, 27; Non-OSA, 136) (Table 4). Qualitative pre-debridement cultures did not vary between groups in terms of incidence of positive culture, polymicrobial status, pathogen type, or bacterial load. Post-
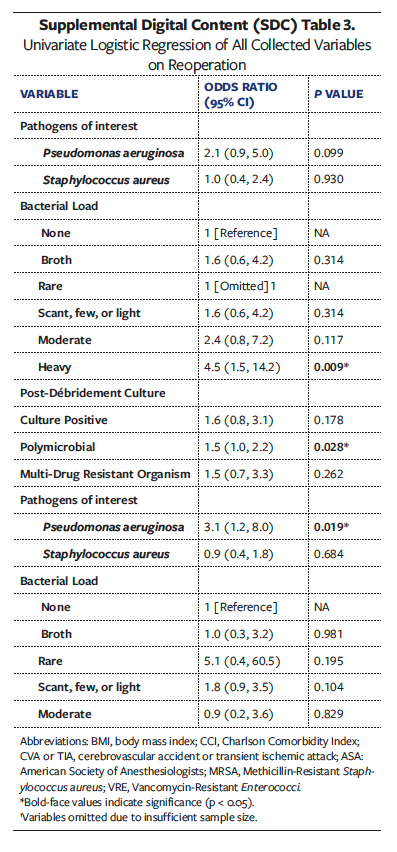
debridement cultures were available for 202 wounds overall (OSA, 32; Non-OSA, 170). On this culture, OSA wounds had a significantly higher incidence of Pseudomonas aeruginosa (n = 7 [21.9%] vs n = 13 [7.7%]; P = .013). Type of postoperative dressing did not vary significantly between groups.
Postoperative complications and long-term outcomes
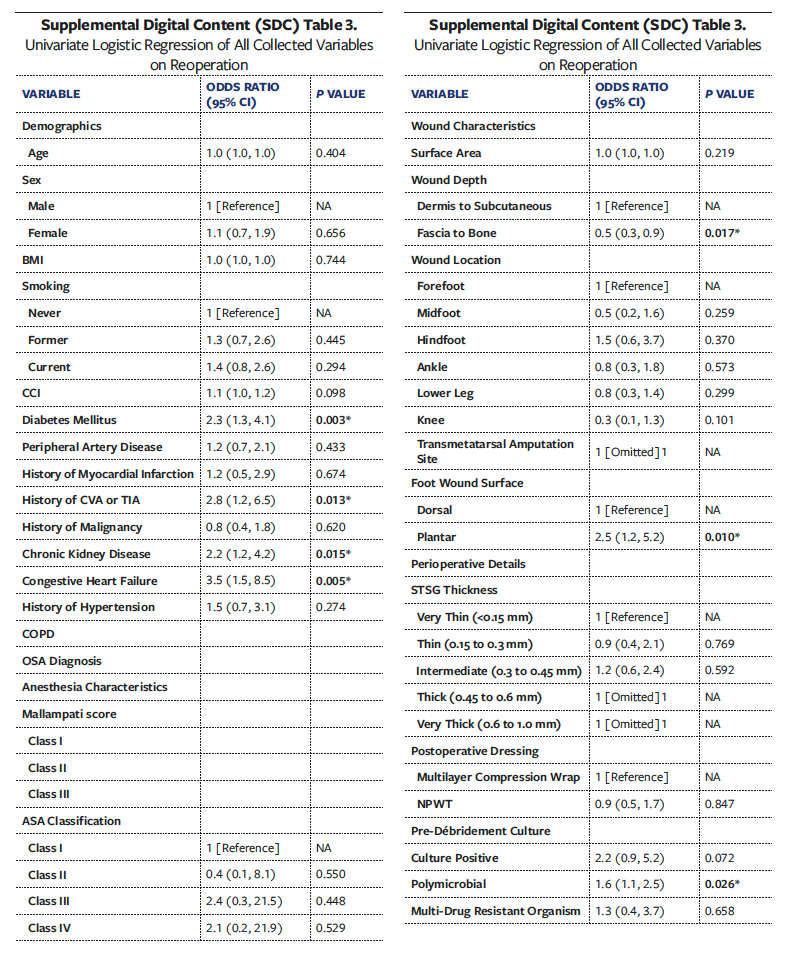
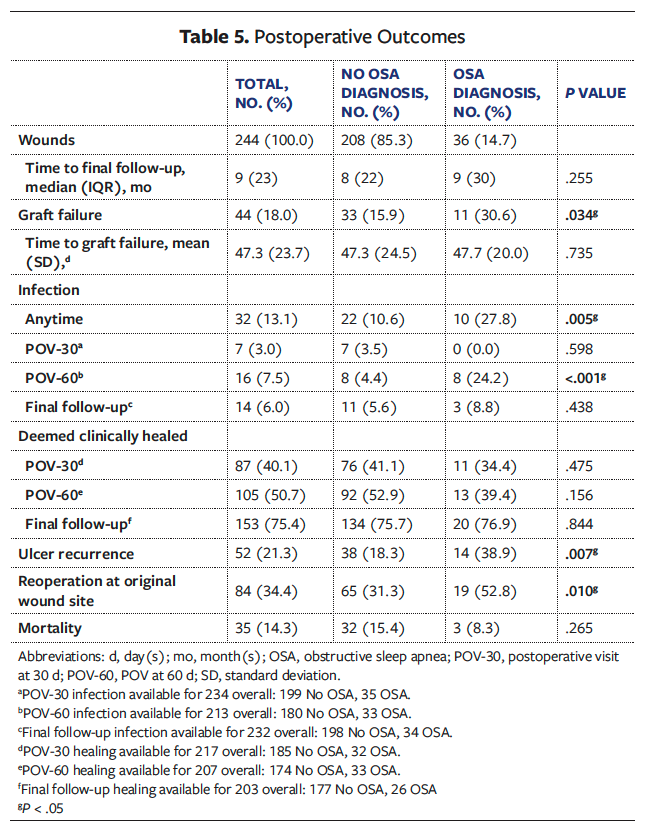
Postoperative outcomes are summarized in Table 5. The OSA group demonstrated significantly higher rates of graft failure compared with the Non-OSA group (n = 11 [30.6%] vs n = 33 [15.9%]; P = .034). Mean (SD) time to graft failure was similar between groups (OSA, 47.7 [20.0] days vs Non-OSA, 47.3 [24.5] days; P = .735). The OSA group experienced significantly higher rates of infection during their entire postoperative course (n = 10 [27.8%] vs n = 22 [10.6%]; P = .005) and at POV-60 (n = 8 [24.2%] vs n = 8 [4.4%]; P < .001). By POV-30, 87 wounds overall were documented as healed (40.1%), which increased to 105 (50.7%) by POV-60 and 153 (75.4%) at final follow-up. There were no statistically significant differences in healing rates between groups. However, by final follow-up the OSA group had significantly higher rates of ulcer recurrence (n = 14 [38.9%] vs n = 38 [18.2%]; P = .007) and reoperations at the original wound site (n = 19 [52.8%] vs n = 65 [31.1%]; P = .010).
Univariate and multivariate analyses
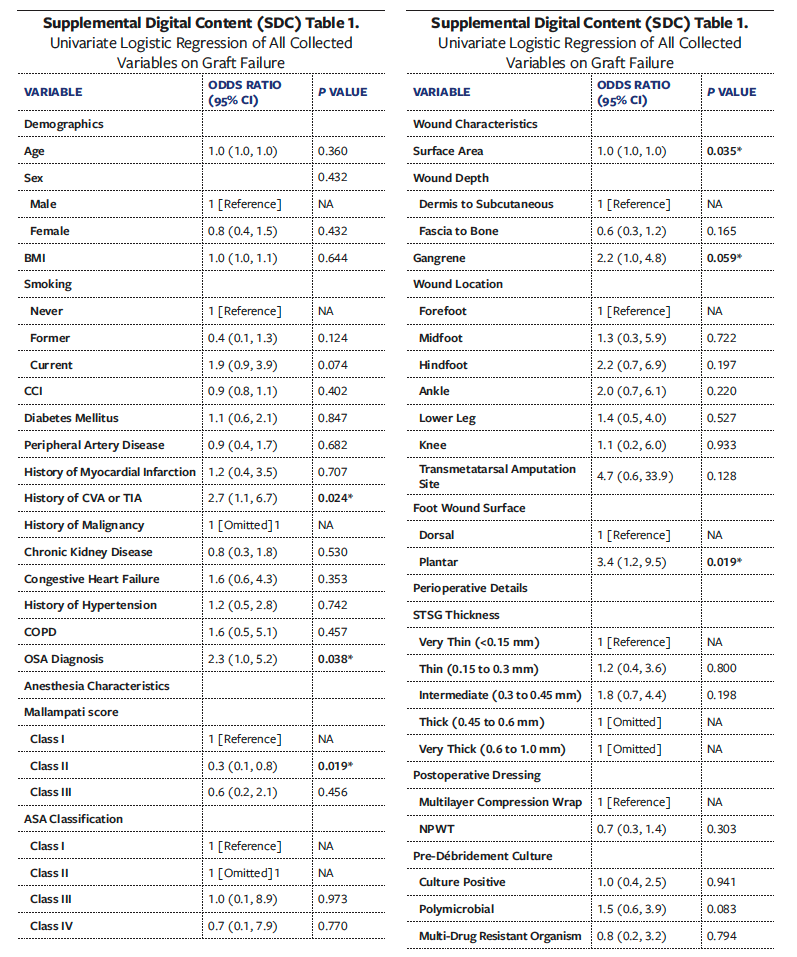
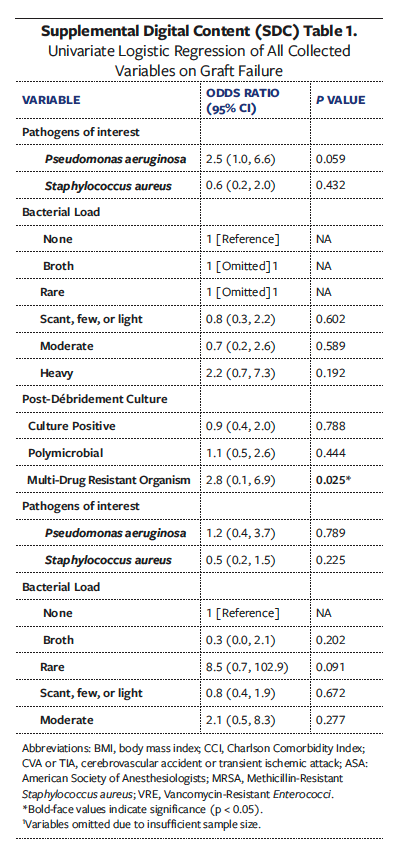
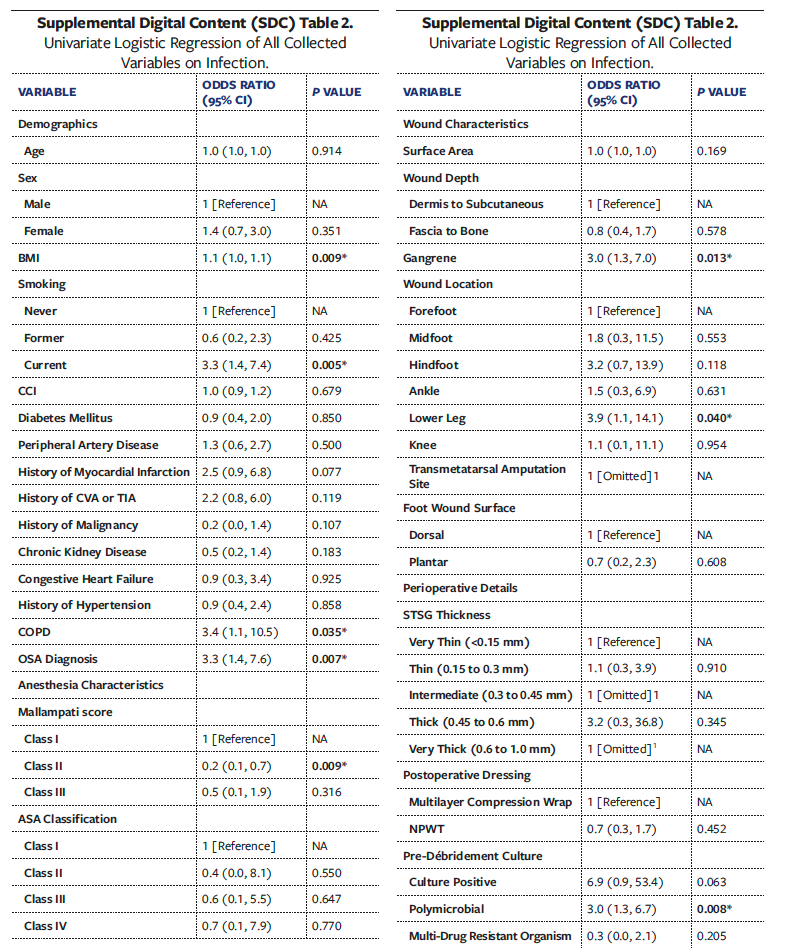
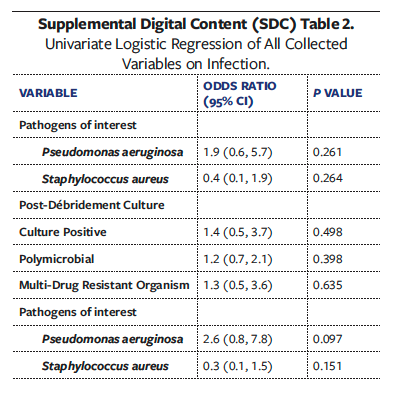
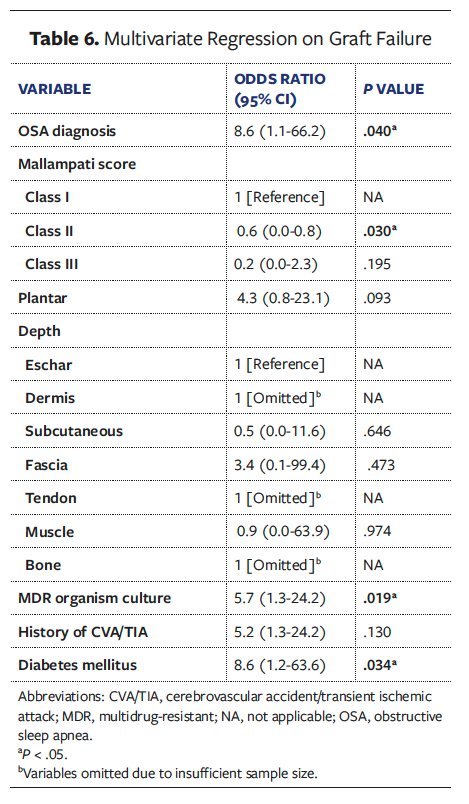
Univariate analyses of all collected variables for graft failure, infection, and reoperation are presented in Supplemental Digital Content (SDC) Tables 1, 2, and 3, respectively. To create multivariate models assessing the independent effect of OSA on graft failure, infection, and reoperation, significant univariate covariates and clinically significant comorbidities were included (Tables 6-8). These models demonstrated that OSA significantly increased the odds of graft failure by 8.6 times (P = .040), of infection by 5.7 times (P = .033), and of reoperation by 3.2 times (P = .027).
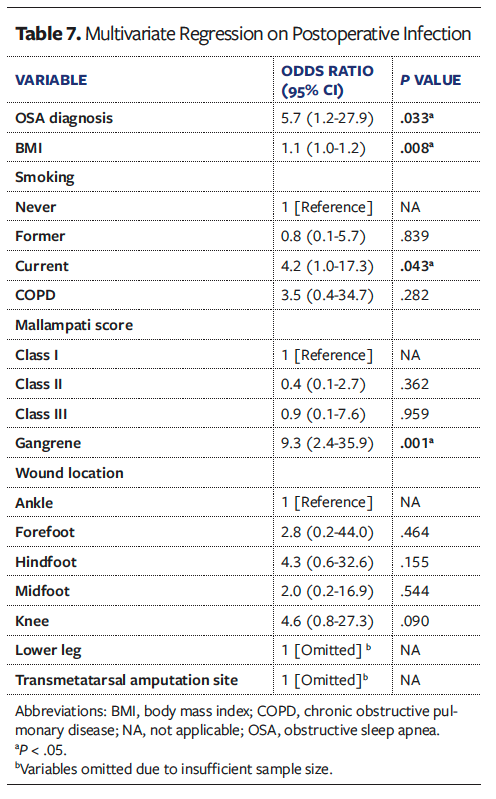
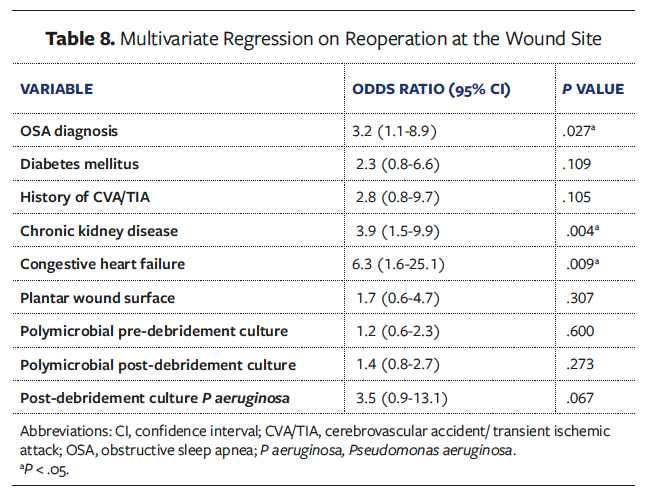
Other comorbidities that remained independent predictors in the final models included DM for graft failure (OR [odds ratio] 8.6, P = .034), higher BMI (OR 1.1, P = .008) and presence of gangrene (OR 9.3, P = .001) for postoperative graft infection, and chronic kidney disease (OR 3.9, P = .004) and congestive heart failure (OR 6.3, P = .009) for reoperation at the wound site.
Discussion
While DM, PAD, obesity, end-stage renal disease (ESRD), and cardiovascular disease are well-known risk factors for poor wound healing, there is a paucity of literature that discusses the effect of OSA in the chronic LE wound population, specifically regarding skin graft outcomes for chronic wound healing. Given the association of OSA with other chronic conditions and its established prevalence in patients with nonhealing wounds, the present study sought to investigative the effect of OSA on LE surgical reconstruction outcomes, specifically STSG. Among 168 patients with chronic LE wounds treated with STSG, a formal diagnosis of OSA was significantly and independently correlated with higher rates of graft failure and postoperative graft infection. Graft failure occurs when there is inadequate nutrient delivery to the graft followed by insufficient revascularization to the wound bed.34,35 In the present study, patients with OSA had a graft failure rate of 30.6%, which was significantly higher compared with patients without OSA (15.9%). After adjustment of significant covariates to graft failure, remaining significant predictors were OSA, Mallampati class II, a positive wound culture for multidrug-resistant organism, and DM. While the reasons for a higher graft failure rate found in the OSA cohort may also be due to a multifactorial interaction of existing comorbidities that impair wound healing, the multivariate analysis in the present study, which controlled for such confounding conditions, confirmed OSA to be independently associated with skin graft failure at an OR of 8.6 times.
It is well-known in the pulmonary literature that OSA is commonly associated with many of the same comorbidities and metabolic conditions36,37 that impair wound healing and could therefore plausibly affect STSG outcomes.4,38-43 Indeed, it has been reported that patients with OSA have an average of 8 other concomitant comorbidities.44 However, the authors of the present study did not observe significant differences in the prevalence of DM, PAD, congestive heart failure, ESRD, and hypertension between the OSA and Non-OSA cohorts, nor were these comorbidities significant on univariate analysis for STSG failure. While the present study is limited in its retrospective design, the authors highlight that OSA is an additional risk factor that should be discussed in this already comorbid population.
The authors of the present study hypothesize that the pathophysiology of OSA and intermittent hypoxia (IH) episodes impair the ability of a skin graft to neovascularize in the acute stages of healing. The authors further postulate that this disruption in capillary formation may reduce the graft’s capacity to sustain itself, ultimately resulting in graft failure. Indeed, OSA has been shown to disrupt wound healing by creating chronic hypoxic conditions, characterized by IH.45,46 IH involves repetitive reoxygenation following hypoxemia, stimulating cycles of ischemia-reperfusion injury that generate reactive oxygen species and activate inflammatory and oxidative stress pathways.13,37,47 STSGs lack an immediate vascular supply, relying entirely on diffusion of nutrients and oxygen during the initial phases of imbibition and inosculation to establish neovascularization. IH could contribute to impaired neovascularization in the early healing phases of a skin graft, compromising its ability to sustain itself and increasing the risk of graft failure.48,49
The study authors’ hypothesis is further substantiated by the findings when they are considered in the context of the relatively more favorable wound characteristics in the OSA group. The OSA group had wounds that were more superficial, were smaller in size, and were treated with thinner STSG—all characteristics that should have led to better graft healing. However, the results demonstrated the opposite. Patients with OSA had smaller wounds that should have favored successful healing compared with larger wounds with deeper exposed structures, which represent a larger healing burden that requires more extensive granulation formation and higher revascularization demands.50,51 Likewise, the use of thinner skin grafts should have conferred a higher graft success rate because thinner skin grafts are capable of increased nutrient diffusion and neovascularization.52 Despite the OSA group having more favorable preoperative conditions, the rate of skin graft failure remained significantly higher among patients with OSA in both univariate and multivariate analyses, which serves to further support the study authors’ hypothesis and may speak to the systemic effect of OSA on wound healing.
The results in the present study also demonstrate that OSA is independently associated with infectious complications after STSG. This finding agrees with existing literature demonstrating that OSA is associated with increased postoperative infection across various procedures.10 Notably, in the present study, contamination with P aeruginosa was significantly higher in the OSA group after debridement. The pathogenicity of P aeruginosa in chronic wounds is well established, and this pathogen is well known to thrive in hypoxic environments in order to adapt to thick biofilm communities.53,54 While no studies directly evaluate whether or not OSA alters the LE chronic wound microbiome, existing literature shows that OSA is significantly associated with alterations to gut, nasal, and upper airway microbiomes.55-57 Given the multiple mechanisms of OSA that lead to increased inflammation and hypoxic environments, the risk of infection—possibly with bacteria that thrive in such hypoxic conditions—may be increased. While anaerobic microbes are difficult to detect in the qualitative cultures used in the present study, future studies may benefit from evaluating the anaerobic microbiome of wounds in patients with OSA.
It is important to note that in the multivariate analyses in the present study, several well-established comorbidities also remained significant predictors of poor STSG outcomes alongside OSA. Specifically, DM significantly increased the risk of graft failure, while higher BMI and presence of gangrene were independent predictors of postoperative infection. In addition, chronic kidney disease and congestive heart failure were independently associated with increased risk of reoperation. These findings align with existing literature describing the deleterious effects of metabolic, vascular, and systemic compromise on wound healing. However, despite adjustment for these comorbidities, OSA remained a strong independent predictor of graft failure, infection, and reoperation, with ORs exceeding those of many traditional risk factors seen in the present study. Thus, it is important to consider OSA not in isolation but as part of a complex, multifactorial burden that affects wound healing in this vulnerable population with multiple systemic comorbidities.
Current plastic surgery and wound healing literature has yet to identify OSA as a significant risk factor for skin graft outcomes in patients with chronic comorbidities. While conditions such as DM, chronic kidney disease, congestive heart failure, and PAD are already recognized high-risk factors, the present study demonstrates that OSA should also be included, particularly for patients undergoing STSG and perhaps other LE reconstruction. Further research should explore the pathophysiological mechanisms of this relationship. Collaborative efforts among plastic surgeons and sleep medicine physicians should be strengthened to manage patients with OSA. Early preoperative screening for OSA, perioperative interventions that involve the role of anesthesia to manage hypoxic episodes, and perioperative control of DM are possible interventions that can be used to improve the management of patients with OSA (Figure).58 This is a new finding with acknowledged limitations, yet the authors of the present study believe it is essential to introduce this topic in the literature and in clinical practice to initiate a meaningful conversation around the effect of OSA on STSG outcomes.
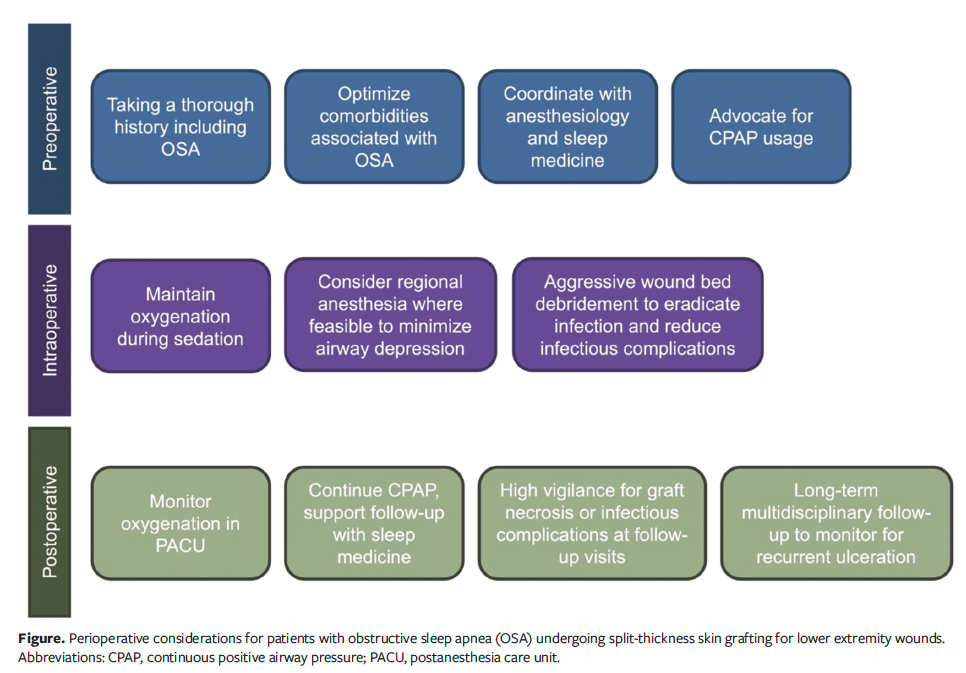
The findings of the present study lay the groundwork for a more structured approach to managing patients with OSA undergoing STSG, and the study authors propose a theoretical algorithm to guide multidisciplinary care (Figure). While this study was not designed to determine whether CPAP treatment mitigates the risks associated with OSA, this remains an important area for future investigation. In this study’s cohort, a subset of patients had documented CPAP use; however, inconsistent documentation regarding adherence and efficacy, along with limited sample size, precluded meaningful subgroup analysis. In future, prospective studies assessing the effect of OSA treatment status (eg, CPAP compliance, optimization of associated comorbidities) may further inform clinical practice. Furthermore, while adjuncts such as NPWT and variations in immobilization protocols are known to influence STSG success and have been well described in the literature, their role in the OSA population warrants closer examination. These factors may have unique implications in this high-risk group. Such efforts will help define which perioperative interventions are most effective and whether patients with untreated OSA may benefit from alternative wound closure strategies. As the field advances toward evidence-based, risk-adapted care models, incorporating OSA into preoperative algorithms may ultimately improve outcomes in complex LE reconstruction.
Limitations
First, this study has several limitations inherent to the nature of the retrospective study design, which relies on the quality of the data collected and consistency of clinical documentation. Second, the sample size of the OSA group was relatively small compared with the Non-OSA group, and given that OSA is widely underdiagnosed, some patients in the Non-OSA group may have had undiagnosed OSA, introducing potential misclassification bias. Additionally, the study authors did not have access to polysomnogram data for patients with OSA, which limits understanding of the severity of OSA, frequency of apnea and hypopnea events, and episodes of desaturation and tissue hypoperfusion. In the statistical analysis, to avoid model overfitting, certain significant covariates were not included in the multivariate regression if they were not clinically relevant to the outcome. Lastly, while 32% of patients in the OSA cohort had documented home use of a CPAP device, treatment adherence and adequacy could not be consistently confirmed in the EMR. Further, this 32% represented a small sample size (n = 8). For these reasons, it was not possible to compare outcomes between patients with treated and untreated OSA with confidence. This represents an important area of ongoing investigation. Despite the limitations, this study demonstrates significant findings on the effect of OSA on wound healing and skin graft outcomes, which are meaningful to both the LE and reconstructive plastic surgery literature. Future research should aim to further glean insights that can guide improved interventions for this patient population.
Conclusion
The present study demonstrates that OSA is an independent predictor of graft failure (OR 8.6; 95% CI, 1.1-66.2) and STSG infection (OR 5.7; 95% CI, 1.2-27.9) in a highly comorbid population with chronic LE wounds. Patients with a known diagnosis of OSA should be carefully managed during the perioperative period to ensure proper oxygenation alongside successful wound healing and skin graft take. Given that OSA is both undertreated and underdiagnosed, preoperative screening efforts to identify patients at high risk for poor wound healing should be undertaken by anesthesia, sleep medicine, and surgical teams to improve outcomes.
Author and Public Information
Authors: Karen R. Li, MD1,2; Rachel N. Rohrich, BS2; Christian X. Lava, MS1; Perry J. Diaz, BS1; Sabrina F. DeLeonibus, MS1; Winnie Li, BS1; Medhat S. Hannallah, MD3; and Christopher E. Attinger, MD2
Affiliations: 1Georgetown University School of Medicine, Washington, DC, USA; 2Department of Plastic and Reconstructive Surgery, MedStar Georgetown University Hospital, Washington, DC, USA; 3Department of Anesthesiology, MedStar Georgetown University Hospital, Washington, DC, USA
Acknowledgments: K.R.L. and R.N.R. contributed equally to this research and serve as co-first authors. All authors sincerely thank Tünay Kuru, MD, MHSA; Ammar Rasul, MD; and Dina B. KiaNoury, MD; from the Division of Pulmonary/Critical Care and Sleep Medicine at MedStar Georgetown University Hospital for their consultation roles to the study design and methodology. The authors also appreciate the assistance of Eshetu Tefera for his role as a consultant in the statistical analysis.
Disclosure: The authors have no financial or other conflicts of interest to disclose.
Ethical Approval: Approval was obtained from the MedStar-Georgetown Institutional Review Board (STUDY00004145) for conduction of a retrospective cohort study.
Correspondence: Christopher E. Attinger, MD; MedStar Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; christopher.attinger@medstar.net
Manuscript Accepted: May 7, 2025
References
1. Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol. 2019;29:8-15. doi:10.1016/j.annepidem.2018.10.005
2. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
3. Wernick B, Nahirniak P, Stawicki SP. Impaired wound healing. In: StatPearls. StatPearls Publishing; 2024.
4. Beyene RT, Derryberry SL Jr, Barbul A. The effect of comorbidities on wound healing. Surg Clin North Am. 2020;100(4):695-705. doi:10.1016/j.suc.2020.05.002
5. Rodrigues BT, Vangaveti VN, Urkude R, Biros E, Malabu UH. Prevalence and risk factors of lower limb amputations in patients with diabetic foot ulcers: a systematic review and meta-analysis. Diabetes Metab Syndr. 2022;16(2):102397. doi:10.1016/j.dsx.2022.102397
6. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687-698. doi:10.1016/s2213-2600(19)30198-5
7. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136-143.
8. Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705-706.
9. Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):144-153. doi:10.1513/pats.200707-114MG
10. Bartolo K, Hill EA. The association between obstructive sleep apnoea and wound healing: a systematic review. Sleep Breath. 2023;27(3):775-787. doi:10.1007/s11325-022-02660-9
11. Chen L, Ma W, Covassin N, et al. Association of sleep-disordered breathing and wound healing in patients with diabetic foot ulcers. J Clin Sleep Med. 2021;17(5):909-916. doi:10.5664/jcsm.9088
12. Maltese G, Fountoulakis N, Drakatos P, et al. Elevated obstructive sleep apnoea risk score is associated with poor healing of diabetic foot ulcers: a prospective cohort study. Diabet Med. 2018;35(11):1494-1498. doi:10.1111/dme.13780
13. Patt BT, Jarjoura D, Lambert L, et al. Prevalence of obstructive sleep apnea in patients with chronic wounds. J Clin Sleep Med. 2010;6(6):541-544.
14. Ramsey ML, Walker B, Patel BC. Full-thickness skin grafts. In: StatPearls. StatPearls Publishing; 2024.
15. Lin C, Liu J, Sun H. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: a meta-analysis. PLoS One. 2020;15(9):e0239236. doi:10.1371/journal.pone.0239236
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
17. Stutz EW, Rondeau B. Mallampati Score. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
18. Braza ME, Fahrenkopf MP. Split-thickness skin grafts. In: StatPearls. StatPearls Publishing; 2024.
19. Johnson TM, Ratner D, Nelson BR. Soft tissue reconstruction with skin grafting. J Am Acad Dermatol. 1992;27(2 Pt 1):151-165. doi:10.1016/0190-9622(92)70164-b
20. Stephenson AJ, Griffiths RW, La Hausse-Brown TP. Patterns of contraction in human full-thickness skin grafts. Br J Plast Surg. 2000;53(5):397-402. doi:10.1054/bjps.2000.3335
21. Abu El Hawa AA, Kim KG, Steinberg JS, et al. Building it from scratch: the team approach to functional diabetic limb salvage. In: Functional Limb Salvage: The Multidisciplinary Team Approach. Springer Nature; 2023:1-11.
22. Attinger CE, Steinberg JS. Functional Limb Salvage: The Multidisciplinary Team Approach. Springer Nature; 2023.
23. Berger LE, Spoer DL, Huffman SS, et al. The role of local flaps in foot and ankle reconstruction: an assessment of outcomes across 206 patients with chronic wounds. Plast Reconstr Surg. 2021. doi:10.1097
24. Cach G, Haffner ZK, Dekker P, et al. Financial toxicity of lower extremity amputation: providing supportive services as part of a multidisciplinary care model. Plast Reconstr Surg. 2023;152(1):200e-201e.
25. Kim PJ, Attinger CE. Negative-pressure wound therapy with instillation: a tool in the multidisciplinary approach to limb function preservation. Plast Reconstr Surg. 2021;147(1S-1):27S-33S.
26. Kim PJ, Attinger CE, Evans KK, Steinberg JS. Role of the podiatrist in diabetic limb salvage. J Vasc Surg. 2012;56(4):1168-1172.
27. Kim PJ, Attinger CE, Steinberg JS, et al. Building a multidisciplinary hospital-based wound care center: nuts and bolts. Plast Reconstr Surg. 2016;138(3S):241S-247S.
28. Kim PJ, Evans KK, Steinberg JS, Pollard ME, Attinger CE. Critical elements to building an effective wound care center. J Vasc Surg. 2013;57(6):1703-1709.
29. Li KR, Lava CX, Neughebauer MB, et al. A multidisciplinary approach to end-stage limb salvage in the highly comorbid atraumatic population: an observational study. J Clin Med. 2024;13(8):2406.
30. Nigam M, Zolper EG, Sharif-Askary B, et al. Expanding criteria for limb salvage in comorbid patients with nonhealing wounds: the MedStar Georgetown Protocol and lessons learned after 200 lower extremity free flaps. Plast Reconstr Surg. 2022;150(1):197-209.
31. Wukich DK, Armstrong DG, Attinger CE, et al. Inpatient management of diabetic foot disorders: a clinical guide. Diabetes Care. 2013;36(9):2862-2871.
32. Zolper EG, Fan KL, Meshkin DH, et al. Reassessing mortality after lower extremity amputation with a multidisciplinary approach focused on function. J Vasc Surg. 2020;72(1):e225-e226.
33. Li KR, Huffman SS, Gupta NJ, et al. Refining a multidisciplinary “vasculo-plastic” approach to limb salvage: an institutional review examining 300 lower extremity free flaps. Plast Reconstr Surg. 2021. doi:10.1097
34. Converse JM, Uhlschmid GK, Ballantyne DL Jr. “Plasmatic circulation” in skin grafts: the phase of serum imbibition. Plast Reconstr Surg. 1969;43(5):495-499.
35. Zarem HA, Zweifach BW, McGehee JM. Development of microcirculation in full-thickness autogenous skin grafts in mice. Am J Physiol. 1967;212(5):1081-1085. doi:10.1152/ajplegacy.1967.212.5.1081
36. Sircu V, Colesnic SI, Covantsev S, et al. The burden of comorbidities in obstructive sleep apnea and the pathophysiologic mechanisms and effects of CPAP. Clocks Sleep. 2023;5(2):333-349. doi:10.3390/clockssleep5020025
37. Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186(5):434-441. doi:10.1164/rccm.201112-2135OC
38. Gleeson M, McNicholas WT. Bidirectional relationships of comorbidity with obstructive sleep apnoea. Eur Respir Rev. 2022;31(164). doi:10.1183/16000617.0256-2021
39. Jarbrink K, Ni G, Sonnergren H, et al. Prevalence and incidence of chronic wounds and related complications: a protocol for a systematic review. Syst Rev. 2016;5(1):152. doi:10.1186/s13643-016-0329-y
40. Mazzotti DR, Keenan BT, Lim DC, et al. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493-506. doi:10.1164/rccm.201808-1509OC
41. Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152(5):1070-1086. doi:10.1016/j.chest.2017.05.009
42. Subramanian A, Adderley NJ, Tracy A, et al. Risk of incident obstructive sleep apnea among patients with type 2 diabetes. Diabetes Care. 2019;42(5):954-963. doi:10.2337/dc18-2004
43. Yalamanchali S, Farajian V, Hamilton C, et al. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350. doi:10.1001/jamaoto.2013.5338
44. Bonsignore MR, Baiamonte P, Mazzuca E, et al. Obstructive sleep apnea and comorbidities: a dangerous liaison. Multidiscip Respir Med. 2019;14:8. doi:10.1186/s40248-019-0172-9
45. Bishop A. Role of oxygen in wound healing. J Wound Care. 2008;17(9):399-402. doi:10.12968/jowc.2008.17.9.30937
46. Rodriguez PG, Felix FN, Woodley DT, et al. The role of oxygen in wound healing: a review of the literature. Dermatol Surg. 2008;34(9):1159-1169. doi:10.1111/j.1524-4725.2008.34254.x
47. Mehta V, Vasu TS, Phillips B, et al. Obstructive sleep apnea and oxygen therapy: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2013;9(3):271-279. doi:10.5664/jcsm.2500
48. Prabhakar NR, Peng YJ, Nanduri J. Hypoxia-inducible factors and obstructive sleep apnea. J Clin Invest. 2020;130(10):5042-5051. doi:10.1172/JCI137560
49. Vural E, Berbee M, Acott A, et al. Skin graft take rates, granulation, and epithelialization: dependence on myeloid cell hypoxia-inducible factor 1alpha. Arch Otolaryngol Head Neck Surg. 2010;136(7):720-723. doi:10.1001/archoto.2010.103
50. Flood MS, Weeks B, Anaeme KO, et al. Treatment of deep full-thickness wounds containing exposed muscle, tendon, and/or bone using a bioactive human skin allograft: a large cohort case series. Wounds. 2020;32(6):164-173.
51. Lee HJ, Kim JW, Oh CW, et al. Negative pressure wound therapy for soft tissue injuries around the foot and ankle. J Orthop Surg Res. 2009;4:14. doi:10.1186/1749-799x-4-14
52. Sun L, Patel AJ. Outcomes of split vs full-thickness skin grafts in scalp reconstruction in outpatient local anaesthetic theatre. Scars Burn Heal. 2021;7:20595131211056542. doi:10.1177/20595131211056542
53. Serra R, Grande R, Butrico L, et al. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti Infect Ther. 2015;13(5):605-613. doi:10.1586/14787210.2015.1023291
54. Costerton JW, Lewandowski Z, Caldwell DE, et al. Microbial biofilms. Annu Rev Microbiol. 1995;49:711-745. doi:10.1146/annurev.mi.49.100195.003431
55. Wu BG, Sulaiman I, Wang J, et al. Severe obstructive sleep apnea is associated with alterations in the nasal microbiome and an increase in inflammation. Am J Respir Crit Care Med. 2019;199(1):99-109.
56. Zhang X, Wang S, Xu H, et al. Metabolomics and microbiome profiling as biomarkers in obstructive sleep apnoea: a comprehensive review. Eur Respir Rev. 2021;30(160).
57. Wang PP, Wang LJ, Fan YQ, et al. Analysis of the characteristics of intestinal microbiota in patients with different severity of obstructive sleep apnea. Sci Rep. 2024;14(1):21552.
58. Vasu TS, Grewal R, Doghramji K. Obstructive sleep apnea syndrome and perioperative complications: a systematic review of the literature. J Clin Sleep Med. 2012;8(2):199-207. doi:10.5664/jcsm.1784