Nonhealing Wound Debridement Using a Finger-Mounted Debridement Tool
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. Chronic wound management consisting of debridement of nonhealing wounds can reduce bacterial bioburden and remove devitalized tissue. However, traditional debridement tools such as curettes, scissors, forceps, and scalpels can result in patient discomfort, particularly in difficult to access areas of tunneling and undermining. Objective. To evaluate the efficacy of a novel finger-mounted debridement tool (FMDT) in the treatment of nonhealing wounds. Materials and Methods. The FMDT was assessed in 11 patients. Patients and clinicians were asked to rate their experience following debridement with the FMDT. Results. The debridement time was 43 ± 14 seconds for an average wound area of 9.06 ± 9.04 cm2 prior to debridement. Clinicians rated ease of use and wound accessibility as 5 (highest rating). No bleeding or minimal amounts of bleeding was observed in 92% of wounds post-debridement. In a survey, 67% of patients reported no pain and 33% reported a pain level of 2 to 6 out of 10 possible. Compared with traditional debridement tools, the FMDT alone was preferred by 56% of patients and the FMDT with traditional debridement tools was preferred by 33%. Of the patients who responded to the survey, 67% reported being less anxious, 78% perceived faster procedure time, and 89% perceived easier wound access. Providers reported that the FMDT was easy to use and the wounds were more accessible than with traditional tools. Conclusion. The FMDT proved to be an effective wound debridement tool that required less time, improved wound accessibility in challenging and uneven areas, and was easier to use than traditional debridement tools.
Common examples of chronic wounds include diabetic ulcers, pressure injuries, and venous leg ulcers.1 Chronic nonhealing wounds can result from nondebridement of bacterial biofilm. Removal of bacterial biofilm and devitalized tissue can enhance wound healing and revitalize the wound healing process. Autolytic debridement uses the body’s own natural wound healing process to remove necrotic tissue. However, for chronic wounds the wound debridement process often requires intervention to initiate wound healing.2
Different types of wound debridement methods have been evaluated, including chemical, enzymatic, biological, surgical or sharp, and autolytic.3 Debridement is the removal of devitalized or necrotic tissue to allow for the healing of the remaining healthy tissue.4 In addition to enhancing wound healing by the removal of devitalized tissue, debridement also helps reduce the risk of infection.
Management of chronic wounds in wound care centers involves surgical, mechanical, and chemical debridement; removal of necrotic tissue and biofilm; prevention of primary infection; nutrition balance; and dressing application.5,6 The soft tissue wound healing process is divided into 4 phases: hemostasis, inflammation, proliferative, and remodeling.6 Granulation tissue is completely formed when (1) a continuous layer extends across the entire wound gap, and (2) the layer of granulation fills the entire wound depth.7
Some additional methods of debridement include pulsed lavage with suction and low-frequency ultrasound debridement.8,9 Medical use of sterile fly larvae (maggot therapy) has also been proven to be effective in certain cases of ulcers.9,10
The standard of care (SOC) treatment includes the use of mechanical or sharp debridement of necrotic tissue and biofilm at the wound site. Although surgical debridement is generally considered the most efficient method when performed by a skilled physician, it may involve excision of some viable tissue along with necrotic tissue.10
Sharp debridement in a clinical setting, although effective and less aggressive compared with surgical debridement, can be quite painful and can have limited access to undermined surfaces.10 A review of randomized controlled trials of wound debridement assessed the superiority of one debridement technique over another.11 While the enzymatic debridement method was found to be better than the saline-soaked procedure, the review found insufficient evidence to support any particular method.
Bahr et al12 conducted a prospective observational evaluation of 60 patients with chronic wounds to test the efficacy of a novel monofilament fiber product. Debridement was found to be effective in 93.4% of the wound debridement sessions, successfully removing debris and slough. The average time spent for each debridement session was 2.51 minutes. Patients reported no pain or slight discomfort during debridement in 45% and 55% of cases, respectively.12
Monofilament fiber debridement pads are associated with reduced treatment time, decreased pain, and reduced cost, and they are more effective than SOC.13,14 In a study of 62 participants with wound size greater than 4 cm2, the mean (SD) time for debridement was 3.5 (2.6) minutes using a monofilament fiber debridement pad.15 Eighty-four percent of patients did not experience an increase in pain during debridement. Significant reductions were observed in necrotic tissue, slough, and debris post-debridement. The clinical investigators were satisfied with use of the monofilament fiber debridement pad and stated that it was effective and easy to use.15
For this study, a new and novel finger-mounted debridement tool (FMDT) was evaluated. The FMDT is a finger-mounted class I medical device intended to remove devitalized, nonviable, and slough tissue from wounds. The proprietary surface on the underside of the device is intended to reduce damage to healthy tissue. The device is also intended to remove biofilm to aid in transitioning chronic wounds to acute wounds. The FMDT can be used to crosshatch necrotic and slough tissue. It incorporates the mobility of the finger, permitting the clinician to reach areas that are inaccessible with the use of traditional debridement instruments.
The present study was conducted to demonstrate the acceptability, feasibility, and effectiveness of the FMDT for chronic wound debridement. To date, no published clinical studies have investigated the use of this type of device for the treatment of chronic wounds. The current study assessed the advantages of using the FMDT for mechanical debridement in comparison with the traditional tools for wound debridement.
Materials and Methods
Study design
This was a nonrandomized, prospective, single-arm study of patients with an established diagnosis of chronic nonhealing wounds requiring SOC wound debridement. The primary objective was to evaluate the effectiveness of the FMDT (DigiTouch Wound Debridement Tool; Medline Industries, LP) for wound debridement in comparison with traditional SOC debridement tools (forceps, scalpels, curette, scissors) and procedures. The FMDT is a new and novel sterile, single-use debridement device designed to fit onto 1 finger to allow the clinician easier dexterity and more precise control during wound debridement (Figure 1).

The study population included 11 patients evaluated during the normal course of clinical operations at AdventHealth Zephyrhills, Zephyrhills, FL. The patients were aged 26 to 84 years, with the majority of patients over 60 years of age. Five of 10 patients were female, and 5 were male. This small investigational pilot study was done to determine the feasibility, effectiveness, and acceptability of using the FMDT device in a larger patient population.
The inclusion criteria for the study included adult patients with an established diagnosis of slow-healing chronic wounds or acute wounds requiring surface debridement. Adult patients with wounds requiring stimulation of the superficial layer to activate the healing process in transitioning from chronic wounds to acute wounds were also included. Only patients who had previous experience with traditional SOC debridement devices (forceps, scalpels, curette, scissors) were included in the study. All patients provided written informed consent 1 week prior to undergoing debridement with the FMDT.
Ethical considerations
The study protocol was approved by the Florida Hospital Tampa institutional review board (IRB) Dactyl Debrider: A Pilot Study (IRBNet#2018-015-1205592). Written consent was obtained from the patients prior to the study. This was done in a private area, by a trained and qualified team member. Patients were asked about participation and recording digital images of the wound during the study. The identity of the patients after collecting and recording the data remained confidential. Patient confidentiality was maintained after data collection
Potential risks of treatment
Although all patients were at risk for morbidity and mortality associated with chronic nonhealing wounds, the risks were minimal in all cases. Potential risks included pain, adverse events related to the use of topical anesthetics, ineffective removal of superficial tissue, allergic reaction to metals used in the debridement device, and anxiety.
Potential benefits of treatment
Potential benefits to the patient included decreased pain and anxiety, an ability to cover a larger surface area in less time, an improved ability to access tunnels and undermining areas and to palpate the area, and an ability to effectively and more precisely crosshatch nonviable or devitalized tissue. The patients did not receive any financial incentive for participating in the study. For health care providers, the FMDT is easier to use than other methods and allows better rapport with the patient. Overall, the benefits considered exceeded the risks.
Study procedure
Digital photographs were taken immediately before and after debridement of the wound with the FMDT in order to assess for skin surface changes. The type of devitalized tissue (slough, biofilm, fibrin, or hypertrophic) in the wound prior to debridement was recorded. Visual observation was also done to confirm effective removal of necrotic tissue slough and biofilm. Patients received either alginate or collagen-based dressings following debridement. One patient also received negative pressure wound therapy.
Provider-collected data
The size and area of the wound were recorded, along with the procedural time and amount of bleeding pre- and post-
debridement. Data were also collected from the clinician who performed the debridement procedure regarding “ease of use” and “accessibility of the wound” using the FMDT. The same clinician treated all patients in the study. These variables were rated on a scale of 0 to 5, with 0 being the worst score and 5 the best.
Patient-collected data
Patients completed a questionnaire post-procedure describing their anxiety, pain level, perceived efficacy, and ease of use with the FMDT compared with their experience with traditional SOC devices. The responses related to patient perception of the FMDT were recorded via phone, using a standardized questionnaire, by personnel unrelated to the patient’s care. Patients were also asked how likely they were to ask for the “FMDT only” or a “combination of FMDT and traditional devices” for any future debridement. The data were analyzed and are presented in the form of descriptive charts, a table, and photographs.
Results
A clinical summary of wound debridement with the FMDT in the 11 patients (12 wounds) included in this study is provided in the Table. The wound types were as follows: 3 venous, 1 venous/surgical, 2 surgical, 2 pressure injuries, 3 laceration/trauma, and 1 diabetic foot ulcer. The average (SD) wound area was 9.06 ± 9.04 cm2 prior to debridement, and the average length of time for the procedure with the FMDT was 43.18 ± 14.02 seconds. The fastest debridement procedure was 26 seconds and the slowest was 75. Bleeding amount was the same pre- and post-procedure for 10 wounds; only 2 wounds had increased bleeding post procedure. The devitalized tissue debrided was slough (8 wounds), biofilm (5 wounds), fibrin (7 wounds), and hypertrophic (1 wound).
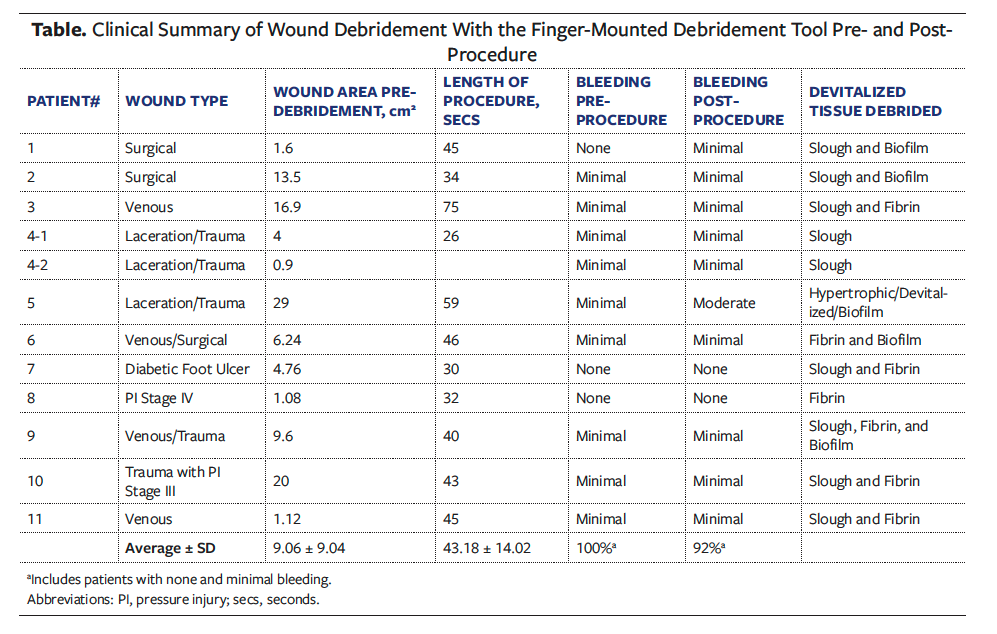
Visual assessment of wounds
The wounds were visually assessed, and photograph images were captured by the clinician pre- and post-debridement with the FMDT (Figure 2). The post-debridement assessment was done after 2 consecutive sessions using the FMDT exclusively. The amount of bleeding and extent of crosshatching of devitalized tissue were observed, and the size of the wound was measured. In most cases, the clinician was able to effectively and precisely crosshatch necrotic debris and tissue, create a bleeding surface for improved wound healing, and reduce the size of the wound. In wounds with hard-to-reach areas, the FMDT afforded better access compared with other debridement instruments.
Clinician responses
Clinician perception while performing debridement with the FMDT was recorded from the following 2 responses: ease of use and accessibility of the wound. Ease of use of the FMDT was rated on a scale of 0 to 5 (0 = “very difficult to use” and 5 = “very easy to use”). For all 11 patients the clinician rated the device ease of use as 5, or very easy to use, in comparison with traditional SOC debridement tools. Accessibility of the wound using the FMDT was also rated on a scale of 0 to 5 (0 = “not accessible at all with this tool” and 5 = “entire wound accessible with this tool”). For all 11 patients the clinician rated the accessibility of the wound as 5, that is, use of the FMDT provided access to the entire wound area. Compared with traditional debridement tools, the FMDT provided better access to the entire wound area with ease and efficiency.
Patient responses
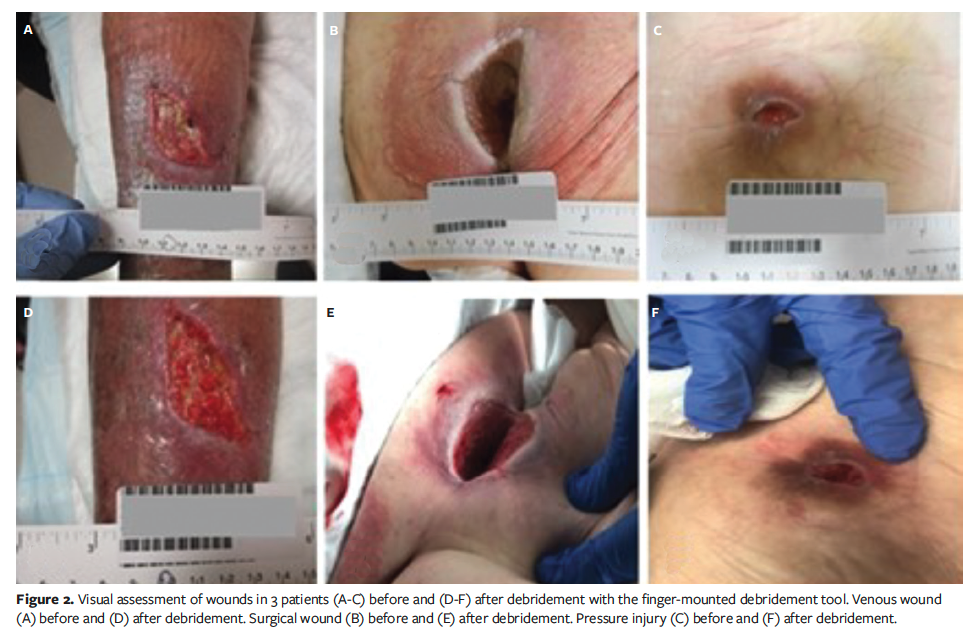
The pain level response of patients undergoing debridement with the FMDT is shown in Figure 3. Patients were asked to rate their pain on a scale from 0 to 10 in reply to the question, “How was your pain level during the procedure with 0 being no pain and 10 the worst pain that you have ever had during a debridement?” Of the 11 patients who underwent debridement with the FMDT, only 9 responded to the survey. Of those, 67% (n = 6) responded that their level of pain was 0 while undergoing the procedure. The 33% of patients (n = 3) who responded with a pain level greater than 0 indicated pain levels of 2, 3, and 6. Overall, patients experienced a very low level of pain during debridement with the FMDT.
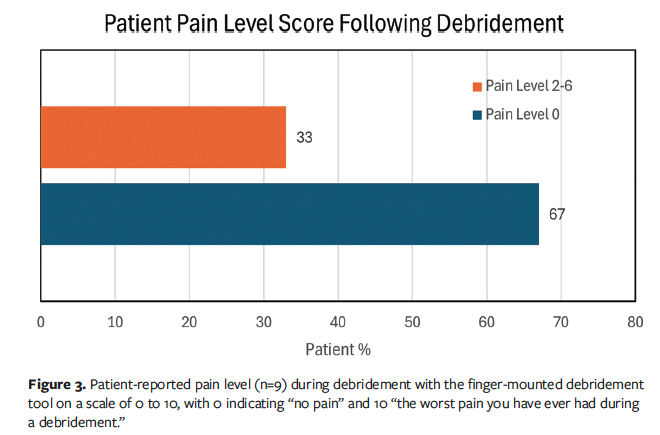
The remaining patient questionnaire responses related to the FMDT debridement procedure compared with traditional debridement tools and previous debridement. These replies, by question, are summarized in Figure 4. Although 9 patients were asked to answer the questions, only 8 patients consistently answered all 4 survey questions.
Question 1, Patient Anxiety Level, read, “How was your anxiety level using the FMDT vs past debridement with traditional debridement tools (curette or scalpels pre-procedure)?” The response choices were: (a) less anxious, (b) more anxious, or (c) just as anxious. Of the patients who received debridement with the FMDT, 67% (n = 6) responded that they were less anxious in comparison to past debridement with a curette or scalpel, 22% (n = 2) replied that they were just as anxious, and 11% (n = 1) responded that they were more anxious (Figure 4A). The patient who was more anxious explained that they did not know what to expect because they were not familiar with the FMDT device. For this patient, they were already familiar with traditional debridement tools such as scissors, scalpels, curettes, and forceps, but the notion of a new procedure made them more anxious.
Question 2, Patient Perceived Speed of the FMDT Procedure, read, “Based on your perception and compared to previous debridement would you say that your procedure was?” The response choices were: (a) faster, (b) slower, or (c) same time. Of the patients who received debridement with the FMDT, none perceived that the new debridement procedure took more time in comparison to previous debridement procedures (Figure 4B). Out of 9 respondents, 78% (n = 7) thought the debridement process was faster with the FMDT and 11% (n = 1) thought that it took about the same time compared with prior debridement procedures using traditional tools. One patient did not respond to the survey question.
Question 3, Perceived Ease of Access and Comparison to Previous Debridement, read, “Based on your perception and compared to previous debridement would you say that your doctor was able to access different and difficult areas on your wound?” The response choices were: (a) easier, (b) more difficult, or (c) equally. All 9 patients who answered the survey question thought the new debridement procedure with the FMDT was easier for the health care provider. In terms of accessing different and difficult areas of the wound, 89% of patients (n = 8) thought the procedure was easier in comparison to traditional debridement tools (Figure 4C). One patient (11%) did not answer the question.
Question 4, Future Device Preference for Debridement, read, “After seeing your medical provider use the FMDT would you prefer your provider to use it on you?” The response choices were: (a) the FMDT only, (b) the FMDT in combination with other tools like: forceps, curettes, scissors, and scalpels, or (c) traditional instruments only: forceps, curettes, scissors, and scalpels. Of the 9 patients who answered the survey questions, 56% (n = 5) preferred the FMDT over the use of traditional debridement instruments (Figure 4D). Of those respondents, 33% (n = 3) said they would prefer a combination of the FMDT and traditional instruments be used by their provider for future procedures. No patient responded that they would prefer the traditional instruments only. One patient (11%) did not respond to the question.
Discussion
Simple single-use wand-shaped or glove-shaped mechanical debridement devices have been evaluated in clinical studies.16 Using a wand-shaped debridement device on 22 patients with 28 wounds resulted in patients experiencing minimal pain, as well as reduced bacterial load in 69% of cases.17 In another study, 23 clinicians were asked to complete a questionnaire comparing a monofilament fiber pad with a handle to their experience using SOC devices.18 Debridement time with the monofilament fiber pad was equal to or shorter than with SOC in 90% of the cases, and the pad was easier to use in 100% of the cases. Clinicians scored debridement efficiency with the monofilament fiber pad as equal to or better than SOC in 67% of cases and as being effective in hard-to-reach areas of the wound.18 In a survey of 1129 participants, both health care professionals and patients reported high levels of satisfaction regardless whether they had previous experience using a monofilament fiber debridement pad.19
The objective of the current study was to assess the acceptability of the FMDT by the patient and the clinician through use of objective visual observations and subjectively through the use of a survey, understand the clinician perspective of the ease of use and effectiveness of wound care with the FMDT, qualitatively and visually assess the difference in wound condition before and after the procedure using the FMDT, and understand the feasibility of using the FMDT in routine wound debridement procedures as a replacement for traditional debridement tools.
The study, which included 11 patients with nonhealing wounds, revealed that the FMDT is acceptable to both the patient and the clinician as a novel wound debridement tool. For 100% of the cases, the providers rated the FMDT as a 5 (best score) for 2 items: ease of use (“easy to use”) and accessibility to the wound (“entire wound accessible with this tool”). The average time needed for the debridement was only 43 seconds, which is faster than with traditional debridement tools, which typically require 1 minute to 5 minutes, per the author’s personal experience. On visual analysis, there was considerable reduction in the average wound area, as well as effective removal of slough, biofilm, and fibrin post-debridement. No bleeding or minimal bleeding was observed in 92% of cases post-debridement.
When asked about pain, 67% (6 of 9) of patients said that the level of pain was 0 (no pain) on a scale of 0 to 10. The highest pain score reported by the 3 patients who reported pain was 6, followed by pain scores of 3 and 2. Patients were less anxious during the debridement procedure with the FMDT, with 67% reporting being less anxious than with traditional debridement. Those who were more anxious added that the anxiety was due to the newness of the tool and not knowing what to expect.
Of the 9 patients who responded to the survey, 89% perceived the wound access to be easier and 78% believed the procedure to be faster than with traditional debridement tools. The use of “FMDT only” for future debridement procedures was preferred by 56% of patients. Another 33% of cases preferred that the FMDT and traditional debridement tools be used in combination. When these categories are combined, 89% of patients preferred the FMDT. None of the patients preferred traditional debridement tools alone.
The FMDT may also have several economic, process-related, and patient-perceived benefits. The use of this device could significantly reduce the debridement time per procedure, providing health care professionals with opportunities to perform more procedures and to care for a greater number of wound care patients. The device developer hopes it will replace traditional sharp debridement as the primary practice and help change the way clinicians approach wound care.
Using the FMDT, fewer tools are necessary per procedure while still achieving coverage of a large surface debridement area. Considering the patient perspective, pre- and postprocedure anxiety should be significantly reduced with this device in comparison to traditional procedures. No debridement tool equivalent to the FMDT is currently being used exclusively by wound care practitioners to debride and prepare wound beds.
Limitations
The current study has limitations. Wound debridement using the FMDT was performed in only 11 patients. To make this research more standardized and rigorous with a focus on quantitative assessment and to further establish these parameters and outcome variables, a future study with more patients is needed. A future 3-arm randomized controlled trial is proposed, with 1 arm representing patients receiving wound debridement treatment with the FMDT, a second arm receiving treatment with traditional debridement tools, and a third arm as the control receiving no intervention for that particular period of time. It should be noted that, like some other mechanical debridement tools, the FMDT occasionally may not allow for debridement of wound edges, and the device functions somewhere between a wound debridement and a wound preparation device.
Conclusion
The results of this study show that use of the FMDT in routine wound debridement is fast, safe, reliable, feasible, and effective for nonhealing wounds. The FMDT was rated as highly favorable by both the clinician and patients in comparison with traditional debridement tools. Overall, patients preferred the FMDT over traditional debridement tools due to less pain, less bleeding, less anxiety, faster speed, and ease of access with the FMDT. This study established the feasibility, effectiveness, and acceptability of the novel device for wound debridement. The FMDT used in routine wound debridement procedures offers a replacement option for traditional debridement tools as well as a new and novel means of treating chronic nonhealing wounds. The FMDT will serve as an additional debridement tool to assist the wound care provider with difficult-to-access areas, time management, biofilm removal, and pain control.
Author and Public Information
Author: Elvis Castillo-Garcia, MD
Affiliation: AdventHealth Zephyrhills, Zephyrhills, FL, USA
Acknowledgments: The author would like to acknowledge Stephen L. Smith, PhD, Senior Medical Writer, Medline Industries, LP, for providing medical writing support in the preparation of this manuscript. The author would also like to acknowledge Grace Furman, BS, Associate Biostatistician, Medline Industries, LP, for providing data analysis.
Disclosure: The author discloses no financial or other conflicts of interest.
Ethical Approval: The study protocol was approved by the Florida Hospital Tampa institutional review board (IRB) Dactyl Debrider: A Pilot Study (IRBNet#2018-015-1205592). Written informed consent was obtained from each patient.
Correspondence: Elvis Castillo-Garcia, MD, 7050 Gall Blvd, Zephyrhills, FL 33541; Elviscastillo1977@gmail.com
Manuscript Accepted: September 25, 2025
References
1. Ammons MCB. Anti-biofilm strategies and the need for innovations in wound care. Recent Pat Antiinfect Drug Discov. 2010;5(1):10-17. doi:10.2174/157489110790112581
2. Atkin L. Understanding methods of wound debridement. Br J Nurs. 2014;23(12):S10-S15. doi:10.12968/bjon.2014.23.sup12.S10
3. Leaper, D. Sharp technique for wound debridement. World Wide Wounds. 2002. http://www.worldwidewounds.com/2002/december/Leaper/Sharp-Debridement.html
4. Laplaud AL, Blaizot X, Gaillard C, et al. Wound debridement: comparative reliability of three methods for measuring fibrin percentage in chronic wounds. Wound Repair Regen. 2010;18(1):13-20. doi:10.1111/j.1524-475X.2009.00555.x
5. Medical Advisory Secretariat. Negative pressure wound therapy: an evidence-based analysis. Ont Health Technol Assess Ser. 2006;6(14):1-38.
6. Nawaz Z, Bently G. Surgical incisions and principles of wound healing. Surgery. 2011;29(2):59-62. doi:10.1016/j.mpsur.2010.11.011
7. Braiman-Wiksman L, Solomonik I, Spira R, Tennenbaum T. Novel insights into wound healing sequence of events. Toxicol Pathol. 2007;35(6):767-779. doi:10.1080/01926230701584189
8. Tan J, Abisi S, Smith A, Burnand KG. A painless method of ultrasonically assisted debridement of chronic leg ulcers: a pilot study. Eur J Vasc Endovasc Surg. 2007;33(2):234-238. doi:10.1016/j.ejvs.2006.09.027
9. Stanisic MM, Provo BJ, Larson DL, Kloth LC. Wound debridement with 25 kHz ultrasound. Adv Skin Wound Care. 2005;18(9):484-490. doi:10.1097/00129334-200511000-00012
10. Wolff H, Hansson C. Larval therapy--an effective method of ulcer debridement. Clin Exp Dermatol. 2003;28(2):134-137. doi:10.1046/j.1365-2230.2003.01226.x
11. Smith F, Dryburgh N, Donaldson J, Mitchell M. Debridement for surgical wounds. Cochrane Database Syst Rev. 2013;2013(9):CD006214. doi:10.1002/14651858.CD006214.pub4. [Update in: Cochrane Database Syst Rev. 2024;(5)5:CD006214. doi:10.1002/14651858.CD006214.pub5]
12. Bahr S, Mustafi N, Hättig P, et al. Clinical efficacy of a new monofilament fibre-containing wound debridement product. J Wound Care. 2011;20(5):242-248. doi:10.12968/jowc.2011.20.5.242
13. Meads C, Lovato E, Longworth L. The Debrisoft(®) monofilament debridement pad for use in acute or chronic wounds: a NICE medical technology guidance. Appl Health Econ Health Policy. 2015;13(6):583-594. doi:10.1007/s40258-015-0195-0
14. Burnett J, Kerr A, Morrison M, Ruston A. An audit to assess the impact of prescribing a monofilament fibre debridement pad for patients with unhealed wounds after six months. J Wound Care. 2021;30(5):381-388. doi:10.12968/jowc.2021.30.5.381
15. Stürmer E, Debus ES, Atkin L. Clinical performance and safety of a debridement pad with abrasive and non-abrasive fibres. J Wound Care. 2024;33(6):408-416. doi:10.12968/jowc.2024.0162
16. Mayer DO, Tettelbach WH, Ciprandi G, et al. Best practice for wound debridement. J Wound Care. 2024;33(Sup6b):S1-S32. doi:10.12968/jowc.2024.33.Sup6b.S1
17. Al-Jalodi O, Serena LM, Breisinger K, Patel K, Harrell K, Serena TE. A novel debridement device for the treatment of hard-to-heal wounds: a prospective trial. J Wound Care. 2021;30(Sup5):S32-S36. doi:10.12968/jowc.2021.30.Sup5.S32
18. Dissemond J, Eberlein T, Bültemann A, et al. A purpose-designed monofilament-fibre pad for debridement of hard-to-reach wounds: experience in clinical practice. J Wound Care. 2018;27(7):421-425. doi:10.12968/jowc.2018.27.7.421
19. Roes C, Calladine L, Morris C. Rapid debridement with monofilament fibre debridement technology: clinical outcomes and practitioner satisfaction. J Wound Care. 2019;28(8):534-541. doi:10.12968/jowc.2019.28.8.534


















