Evaluation of A Multilayer Antimicrobial Foam Dressing Indicated for Use on Surgical Incisions
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. Many orthopedic procedures are performed every year in the United States on a population deemed low risk for surgical site infections (SSIs). To avoid wound complications, an appropriate dressing must be used that includes features such as conformability and antimicrobial properties. Objective. To evaluate use of a multilayer antimicrobial surgical incision dressing in patients at low risk of SSI . Methods. Data were collected from 8 hospitals in the United States. All patients involved were at low risk of infection following the surgical procedure, and the dressings were used prophylactically. Data were collected using a standardized questionnaire; respondents completed 1 survey per patient about product application, performance of dressing, and continued use. Results. Out of the 54 responses, 83% of surgeries (n = 45) were orthopedic procedures. The dressing was well received, with 91% of clinicians stating that the ease of application met, exceeded, or greatly exceeded their expectations. Seventy percent of respondents stated that the dressing’s ability to conform to body contours exceeded or greatly exceeded their expectations, and 96% of clinicians stated they would like to continue using the dressing. Conclusion. The dressing demonstrates excellent conformability and other required features of a postoperative dressing in a patient population at low risk of SSI.
Many orthopedic procedures, such as knee and hip replacements, are performed every year. Data from the 2000-2014 United States (US) National (Nationwide) Inpatient Sample combined with US Census Bureau data were used to predict that 652,000 total hip arthroplasties (THAs) and 1.272 million total knee arthroplasties (TKAs) would be performed in 2025.1 Generally, these surgical wounds are classified as class 1 (clean) or class 2 (clean-contaminated) as per the Surgical Wound Classification framework.2 Clean is defined as “an incision in which no inflammation is encountered in a surgical procedure, without a break in sterile technique, and during which the respiratory, alimentary, and genitourinary tracts are not entered.” Clean-contaminated is defined as “an incision through which the respiratory, alimentary, or genitourinary tract is entered under controlled conditions but with no contamination encountered.”2 Surgeries are identified as lower risk if the patient has an American Society of Anesthesiologists (ASA) score of 1 or 2.3
Surgical site infections (SSIs) are associated with increased length of stay, as well as higher readmission rates, reoperation rates, and morbidity and sometimes, patient mortality.4,5 It has been estimated that SSIs affect 2% to 4% of patients who undergo inpatient surgical procedures in the United States.6 To avoid wound complications after these surgeries, an appropriate dressing must be used.7 Requirements of the “ideal” dressing for low-risk postoperative incision wounds are as follows: flexible, well fixed to the patient on application, absorbent, skin protective, waterproof, and able to eliminate dead space where necessary.8
In terms of TKA, another recommended wound closure–related intervention is the use of silver-impregnated dressings, which are known to decrease the rate of wound complications compared with standard dressings, reduce the amount of dressing changes, and reduce the risk of SSI.9 This may be due to silver having broad-spectrum bactericidal activity; it is a heavy metal antiseptic.10 Silver-impregnated dressings are also recommended for THA because they can be used for a longer period of time before the first dressing change compared with a conventional dressing, which reduces the need for postoperative intervention.11
The purpose of this study was to evaluate the Allevyn Ag+ surgical dressing (Smith + Nephew; hereafter “multilayer antimicrobial surgical incision dressing”), which incorporates several other technologies proprietary to the same manufacturer. This dressing features ComfortSTAY technology, which is a gentle silicone adhesive designed to maintain 7 days of wear that also minimizes pain and trauma on removal. The dressing also securely fits body contours, aiding patient comfort in wear due to its HighFLEX technology. As well as being showerproof, the ExuLOCK layer protects the skin from exudate leakage. A silver foam layer provides effective antimicrobial action while still being breathable, and the dressing guarantees sustained antimicrobial activity over 7 days on a broad range of wound pathogens.
Methods
Patient population
Data were collected from a total of 8 hospitals in the United States. In total, these sites had 2258 licensed beds, and the geographic classification was 50% urban and 50% rural. Urban sites had a range of 82 to 709 beds, and rural sites had a range of 110 to 372 beds. Survey respondents were interested in evaluating the antimicrobial surgical dressing because they had previously used other dressings by the same manufacturer and in many cases were users of competitor antimicrobial dressings. All patients involved in the evaluation were at low risk of infection following the surgical procedure (based on wound classification and ASA score); thus, the antimicrobial dressings were applied prophylactically. Individual comorbidities were not the focus of this analysis and therefore were not collected in detail. Instead, patients were assessed using surgical wound classification and ASA grade, both of which serve as validated indicators of patient condition and perioperative risk. These measures allowed the study investigators to account for underlying health status in a standardized manner.
Ethical approval
As this project was a product evaluation of clinician opinions, IRB approvals were not needed, nor was patient consent. No patient identifiable data were collected.
Data collection surveys
Data were collected digitally using Snap Surveys Limited SNAP XMP software (Snap Surveys, Ltd) and stored securely on a US-based server in compliance with ISO 27001. This software was used to create a standardized questionnaire to collect responses and to analyze and collate the data into a detailed report of outcomes. No sensitive or identifiable patient data were collected.
Outcomes
Respondents completed 1 survey per patient and answered questions about surgical procedure, surgical wound classification, and patient risk factors (ASA physical status classification).
Additionally, respondents were asked how the dressing met expectations in terms of performance. The dressing was evaluated based on the ease of application, conformity to body contours, the size of the dressing, whether the dressing stayed in place, and the overall dressing performance.
Ease of application was assessed using a structured survey in which respondents rated performance on a 5-item scale. Respondents were also asked whether they would continue to use the dressing and were asked to select its most beneficial feature, with “ease of application” included as one of the predefined options. The authors of this study recognize that this measure reflects user perception rather than an objective metric, but the authors believe it provides meaningful insight into clinical usability. Finally, respondents were asked how the multilayer antimicrobial surgical incision dressing compared with the dressing they were currently using.
Results
Product application
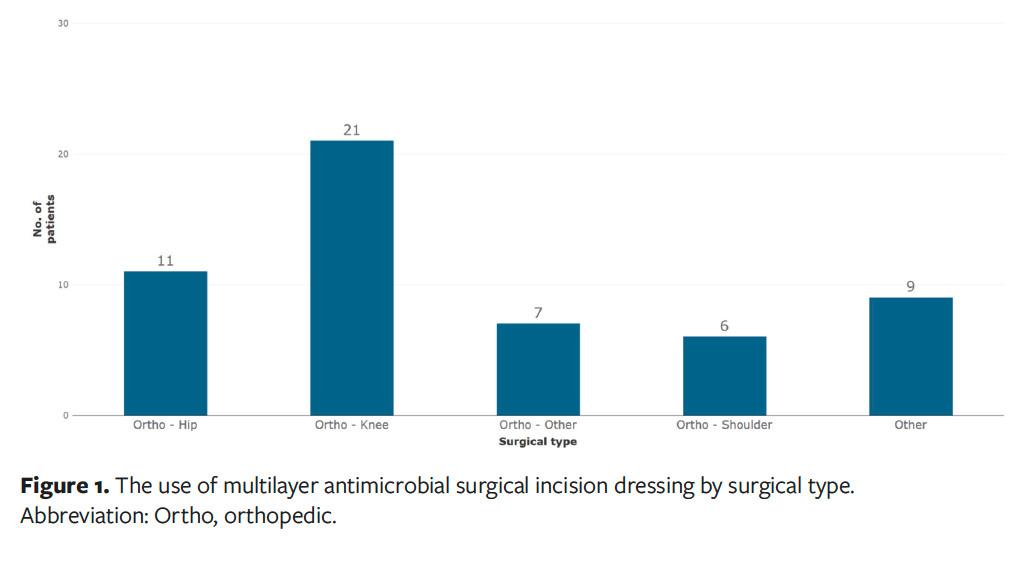
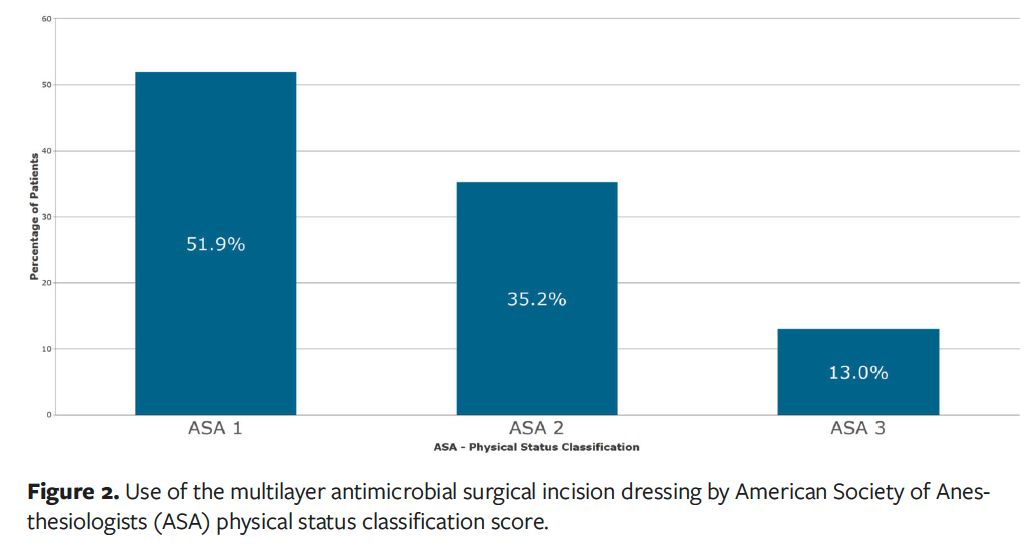
A total of 54 respondents submitted an evaluation form, with 83% of surgeries being orthopedic procedures (n = 45), which included a high proportion of knee surgeries (n = 21) as well as hip surgeries (n = 11), shoulder surgeries (n = 6), and other orthopedic surgeries (n = 7). The remaining 9 respondents had patients categorized as “Other,” which includes neurology (n = 4), cesarean delivery (C-section) (n = 2), trauma (n = 1), urology (n = 1), and general (n = 1) (Figure 1). All these surgeries were low risk, either class 1 (clean, 89% [n = 48]) or class 2 (clean-contaminated, 11% [n = 6]). Fifty-two percent of patients (n = 28) had an ASA physical status classification score of 1, which is a healthy patient with no significant medical history, 35% (n = 19) had an ASA score of 2, meaning they had a mild systemic disease, and 13% (n = 7) had an ASA score of 3 due to severe systemic disease (Figure 2).
Performance of dressing
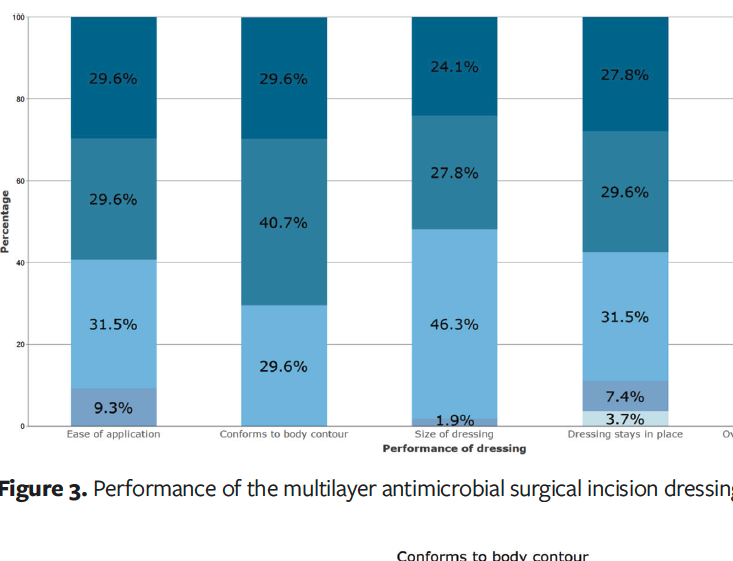
The dressing was well received, with 91% of clinicians (n = 49) stating that the ease of application met, exceeded, or greatly exceeded their expectations. Seventy percent of respondents (n = 38) stated that the dressing’s ability to conform to body contours exceeded or greatly exceeded their expectations, with the remaining clinicians stating that the dressing met expectations. Clinicians were satisfied with the size of the dressing, with 98% (n = 53) stating the size of the dressing met, exceeded, or greatly exceeded their expectations. They were also satisfied with the ability of the dressing to stay in place, with 89% (n = 48) stating that this factor met, exceeded, or greatly exceeded their expectations. In summary, 93% of clinicians (n = 50) stated that the overall dressing performance met, exceeded, or greatly exceeded their expectations (Figure 3).
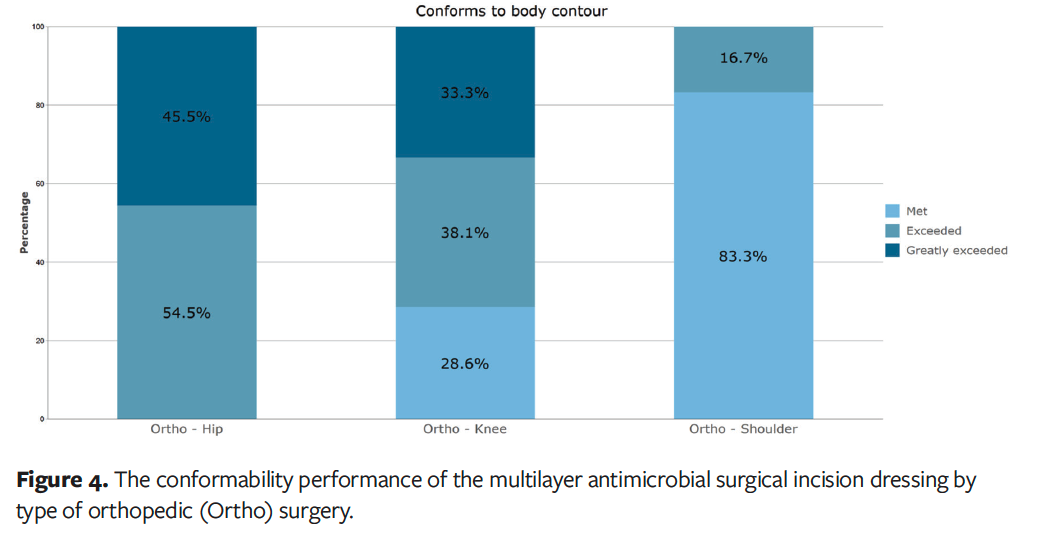
In terms of conformability to body contours for specific orthopedic surgical procedures, over 70% of clinicians who used the dressing after orthopedic knee procedures (n = 15) and 100% of clinicians who used it after orthopedic hip procedures (n = 11) stated that the dressing exceeded or greatly exceeded their expectations (Figure 4). The dressing’s ability to conform to body contours was rated as the most beneficial feature by clinicians (Figure 5).
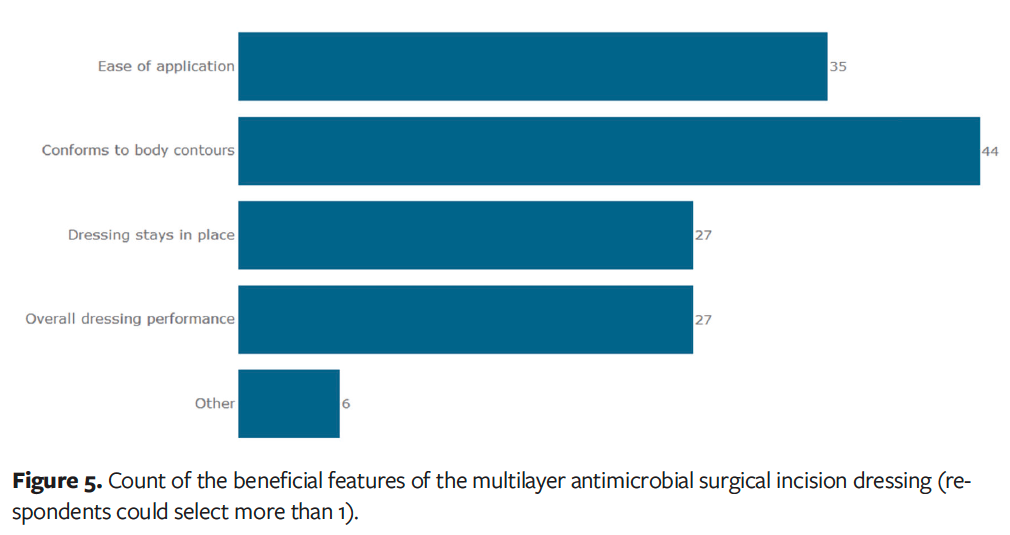
Comparison and continued use
When asked to compare the multilayer antimicrobial surgical incision dressing with other dressings they have used, 63% of clinicians (n = 34) stated the dressing was better than their previously used surgical dressing (Figure 6). Fifty-two clinicians (96%) stated they would like to continue using the dressing.
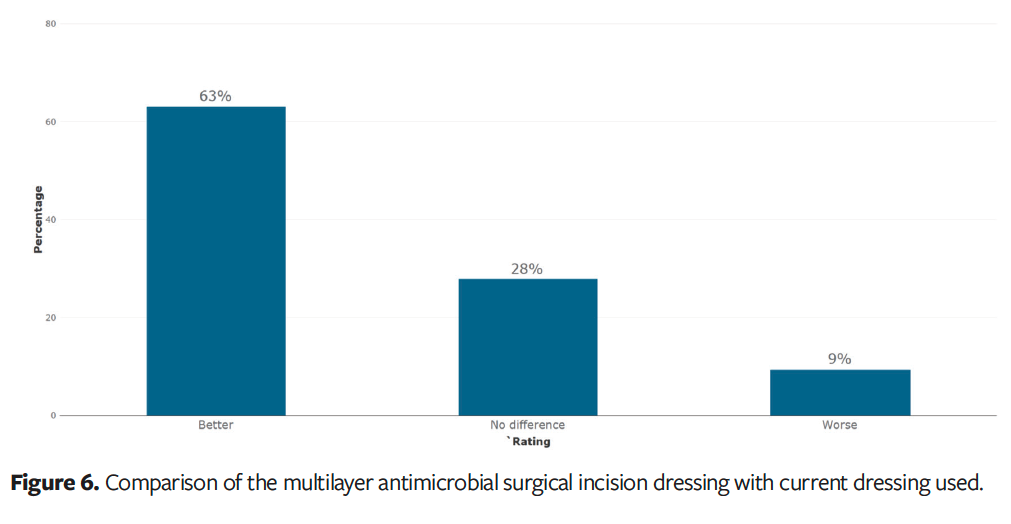
Discussion
Any type of wound, including a surgical incision, in an anatomical location that allows for a large degree of range of motion (eg, knee, shoulder, elbow) can be challenging to manage. In the case of postoperative surgical incisions, it is imperative that the wound be protected from outside sources of contamination during the initial phases of healing. If the dressing is not applied correctly, it does not create an effective barrier between the surgical incision and outside contaminants, which can increase the risk of infection and potentially increase the need for more frequent dressing changes.
The ease of dressing application affects surgical workflow efficiency. If the dressing is difficult to apply, it can be frustrating to the staff, which often leads to incorrect and inconsistent dressing application. Additionally, good dressing conformability can often lead to reduced costs. When a dressing does not conform well to the patient, wear time is reduced and dressing change frequency is increased.
As demonstrated in the results of the present study, the multilayer antimicrobial surgical incision dressing is conformable and reliable, allowing health care professionals to maintain a protected environment to optimize the patient’s ability to heal. These initial results should provide surgical teams with confidence that this dressing can provide a reliable barrier to support optimal postoperative wound healing in a low-risk patient population.
Results suggest that the antimicrobial dressing is suitable for postoperative incisions and that it will support teams in meeting some recommendations from the Delphi study consensus statements on TKA and THA.9,11 The TKA consensus statements suggest that a silver dressing be used,9 which is achieved by the features of the antimicrobial dressing evaluated in the present study. However, the antimicrobial and antiseptic properties of the dressing were not a measured outcome of the present study. Any mention of antimicrobial or antiseptic characteristics in the current study was intended only as contextual background; each dressing contains 1.9 mg/cm2 to 3.0 mg/cm2 of silver. The consensus statements on TKA also highlight the importance of dressing the incision in semiflexion; the positive results around conformity suggest the antimicrobial surgical incision dressing is suitable for use post closure.9
When patients are recovering from surgery, it is imperative that they be able to continue with their lives as they would have done previously.
Limitations
This study has several limitations. One limitation is bias due to survey recruitment, because the people who responded were already customers of the dressing manufacturer and had previously used other dressings made by the same manufacturer. Additionally, the survey was completed by health care professionals, which meant that patient experiences were not captured. The individual who placed the dressing completed the survey. In the operating room, surgical dressings may be applied by a variety of trained clinicians. Therefore, because dressing application responsibilities vary by setting, the specific clinician role for each application was not captured; rather, the survey reflects the experience of the provider performing the application. Tissue adhesive-related injuries were not specifically addressed in the survey. Respondents were asked to compare the multilayer antimicrobial surgical incision dressing with their current surgical dressing (eg, island dressing, tape), to report patient pain level on dressing removal, and to indicate whether they would continue to use the antimicrobial surgical incision dressing. Although these measures do not directly capture adhesive-related skin injury, they do provide insight into patient tolerance and product performance. Additional studies with more clinicians, with both patient and clinician follow-up and to assess factors not assessed herein, are needed.
Conclusion
The multilayer antimicrobial surgical incision dressing evaluated in this study demonstrates excellent conformability and other required features of a postoperative dressing in a patient population at low risk of SSI. The majority of clinicians who responded to the survey indicated that they would continue to use the dressing.
Author and Public Information
Authors: Stephanie Constable, BSN, RN, CWOCN1; Jacquelyn Bergo, MBA, MSN, RN, PHN, CCRN2; Sarah Tuttle, BSc2; and Jennifer Gale, BA2
Affiliations: 1Wound Care and Ostomy United Hospital Center , Bridgeport, WV, USA; 2US Market Access Department Smith + Nephew Inc, Fort Worth, TX, USA
Acknowledgments: The authors would like to extend their thanks to all the sites that took part in this evaluation. In addition, they would like to thank Amy Glasswell for support with manuscript preparation.
Author Contributions: All authors contributed to the study conception and design, material preparation, data collection, and analysis. The manuscript was written and reviewed by all authors, and all authors read and approved the final manuscript.
Disclosure: S.C. is a paid consultant of Smith + Nephew. J.B., S.T., and J.G. are employees and shareholders of Smith + Nephew.
Ethical Approval: As this project was a product evaluation of clinician opinions, IRB approvals were not needed, nor was patient consent. No patient identifiable data were collected.
Correspondence: Jennifer Gale, 101 Hessle Road, Hull, HU3 2AH; Jennifer.gale@smith-nephew.com
Manuscript Accepted: October 27, 2025
References
1. Singh JA, Yu S, Chen L, Cleveland JD. Rates of total joint replacement in the United States: future projections to 2020-2040 using the National Inpatient Sample. J Rheumatol. 2019;46(9):1134-1140. doi:10.3899/jrheum.170990
2. Kamel C, McGahan L, Polisena J, Mierzwinski-Urban M, Embil JM. Preoperative skin antiseptic preparations for preventing surgical site infections: a systematic review. Infect Control Hosp Epidemiol. 2012;33(6):608-617. doi:10.1086/665723
3. Hendrix JM, Garmon EH. American Society of Anesthesiologists Physical Status Classification System. StatPearls [Internet]. StatPearls Publishing; 2025. https://www.ncbi.nlm.nih.gov/books/NBK441940
4. Centers for Disease Control and Prevention, National Healthcare Safety Network. Surgical Site Infection Event. 2025. https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
5. Shambhu S, Gordon AS, Liu Y, et al. The burden of health care utilization, cost, and mortality associated with select surgical site infections. Jt Comm J Qual Patient Saf. 2024;50(12):857-866. doi:10.1016/j.jcjq.2024.08.005
6. Agency for Healthcare Research and Quality, Patient Safety Network. Surgical Site Infections. 2019. Updated: September 15, 2024. https://psnet.ahrq.gov/primer/surgical-site-infections. Accesesed: 29/11/2025.
7. Bredow J, Hoffmann K, Oppermann J, Hellmich M, Eysel P, Zarghooni K. Evaluation of absorbent versus conventional wound dressing. Dtsch Arztebl Int. 2018;115(13):213-219. doi:10.3238/arztebl.2018.0213
8. Morgan-Jones R, Bishay M, Hernández-Hermoso JA, et al. Incision care and dressing selection in surgical wounds: findings from an international meeting of surgeons. Wounds International. 2019. https://woundsinternational.com/consensus-documents/incision-care-and-dressing-selection-surgical-wounds-findings-international-meeting-surgeons/
9. Ainslie-Garcia M, Anderson LA, Bloch BV, et al. International Delphi study on wound closure and dressing management in joint arthroplasty: part 1: total knee arthroplasty. J Arthroplasty. 2024;39(4):878-883. doi:10.1016/j.arth.2023.12.032
10. Yousefian F, Hesari R, Jensen T, et al. Antimicrobial wound dressings: a concise review for clinicians. Antibiotics (Basel). 2023;12(9):1434. doi:10.3390/antibiotics12091434
11. Ainslie-Garcia M, Anderson LA, Bloch BV, et al. International Delphi study on wound closure and incision management in joint arthroplasty part 2: total hip arthroplasty. J Arthroplasty. 2024;39(6):1524-1529. doi:10.1016/j.arth.2024.01.047
















