Efficacy of Cellular and/or Tissue-Based Product Applications on all Non-Pressure Injury Chronic Wound Types in a Medicare Private Practice Model
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. This retrospective analysis is a derivative cohort study based on a prior retrospective investigation by this author group. Objective. To assess the effect of the number of cellular and/or tissue-based product (CTP) applications on healing outcomes and wound area reduction (WAR) rates in patients with chronic wounds of multiple etiologies. Methods. Data from a multicenter private wound care practice electronic health record database were analyzed for Medicare patients receiving CTPs from January 2018 through December 2023. Wound treatments were administered in nursing homes (4.77%), private clinics (80.11%), and home settings (15.65%), excluding hospital outpatient department settings. This retrospective analysis evaluated WAR and closure rates following each CTP application. Results. A total of 446 wounds were included in the analysis, comprising 123 diabetic foot ulcers (DFUs), 134 venous leg ulcers (VLUs), 62 surgical wounds, 51 trauma wounds, and 76 other chronic wounds. Significant reductions in average wound areas (cm²) were observed after completing the CTP application series (ie, ≤10 CTPs within 16 weeks) for all chronic wounds (P < .001). Further, there were more total healed wounds noted for the chronic surgical and trauma wounds compared with DFUs and VLUs. Conclusion. This retrospective real-world analysis of Medicare patients undergoing CTP therapy in conjunction with standard of care for chronic trauma and surgical wounds demonstrates substantial reductions in wound area following completion of a CTP application series. Findings from this study may guide governing bodies regarding CTP best practice recommendations in the treatment of chronic wounds of various etiologies.
Introduction
Chronic wounds, also termed “nonhealing wounds” (NHWs), affect millions of individuals and pose a significant burden on health care systems globally. Chronic NHWs are often defined as wounds that are unable to exhibit the normal healing cascade within a timely manner, and they can be classified into various etiologies, including diabetic foot ulcers (DFUs), venous leg ulcers (VLUs), surgical wounds, pressure injuries, and trauma wounds.1 Disruption of the normal wound healing stages can result from one or several impairments, including infection, malnutrition, a lack of perfusion, oxygenation, or off-loading. NHWs are often characterized by prolonged inflammation, persistent infection, and inadequate tissue regeneration. Chronic wounds also increase patients’ risk of morbidity, diminish patients’ quality of life, and are often associated with societal discrimination, limited mobility, and reduced productivity.1
Further, chronic wounds are often attributed to continued clinical challenges due to prolonged healing times, increased risk of infection, and substantial health care costs. The financial burden of extensive treatments for chronic wounds remains significant. In fact, the total Medicare cost of treating all wounds ranged from approximately $28.1 billion to $96.8 billion in the United States in 2014.2 Traditional standard of care (SOC) modalities in conjunction with cellular and/or tissue-based products (CTPs) are becoming much more common as a means to heal or close NHWs, with the goal of increasing the number of closed wounds while decreasing the time to closure. This advanced wound healing strategy has the possibility to improve patient quality of life while decreasing the number of days of therapy.
Current literature supports the use of CTPs in providing chronic wounds with a source of extracellular matrix scaffolding, growth factors, and, in some cases, cells, to promote tissue regeneration, angiogenesis, and ultimately reepithelialization.3-5 Multiple publications have shown that, compared with conventional wound healing methods, CTPs reduce healing time in chronic wounds, primarily DFUs.6-12 The current evidence underscores the potential of CTP-based therapies to address critical challenges in wound management, offering a promising adjunct to conventional treatments.13 However, there is limited evidence-based literature regarding the efficacy of CTPs in chronic wounds that are not DFUs or VLUs.9,14 Comprehensive retrospective studies are needed to evaluate the real-world effectiveness and safety of CTPs in the treatment of chronic wounds of various etiologies.
The primary objective of the current research study was to evaluate the effectiveness of different CTPs in promoting wound area reduction (WAR) in chronic surgical and trauma wounds. This cohort study was based on the methodology established in a retrospective investigation by Carpenter et al.14 By systematically comparing the outcomes associated with various CTP treatments, the current study aimed to identify the most efficacious strategies for accelerating wound healing with the goal of improving overall patient outcomes. Through this investigation, the study authors hope to contribute to the growing body of knowledge on advanced wound care technologies for the treatment of chronic surgical and trauma wounds.
Methods
Data source and definitions
The electronic health record (EHR) database of a private wound practice in rural Louisiana and Mississippi was used to review all patients with Medicare insurance. Data from those patients receiving CTP applications between January 1, 2018, and December 31, 2023, were used for analysis. Wound treatment occurred in outpatient clinics, nursing home facilities, and patients’ homes. Accounts receivable reports were obtained for identification of International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnosis codes. Patient wound etiologies included DFUs, VLUs, trauma, and surgical. Pressure injuries were excluded from this analysis. Wound etiologies were further verified through accounts receivable reports documented within the EHR. An accounts receivable report was completed to identify patients who received CTP applications between January 1, 2018, and December 31, 2023, and to identify wound sizes at each CTP application. Demographic data and wound characteristics were obtained through manual chart review.
Patients who underwent CTP applications initially underwent SOC treatment for at least 30 days. Patients with less than 50% WAR despite receiving 30 days of SOC treatment qualified for CTP applications. A CTP application series consisted of 10 or fewer CTP applications within 16 weeks. These patients also met the 2024 Local Coverage Determination (LCD) criteria established by Medicare Administrative Contractors (MACs) for chronic wounds to be eligible for CTP treatment. Patients who received fewer than 10 CTP applications were included in the analysis if their wounds healed before completing a 10-application series.
DFUs were characterized as wounds located on the ankle or foot in patients diagnosed with diabetes. VLUs were defined as wounds on the lower extremities in patients diagnosed with venous insufficiency. Trauma wounds were defined as those wounds caused by trauma etiologies. Surgical wounds were defined as wounds of iatrogenic etiology with disruption in external operations. Lastly, other chronic wounds were those of undefined etiology and were documented as “other” throughout the CTP series. For all chronic wounds, chronic was defined as having a duration of 4 weeks or longer to initiating the CTP application series.
Initial wound measurement referred to the wound size (area) at the first CTP application, and the final wound measurement was the size at the last CTP application or 1 week thereafter. Wound areas were obtained by manual measurements with standardized rulers throughout all clinical settings. Wounds were classified as healed if they achieved a 99% to 100% WAR rate based on EHR documentation requirements. Parameters for healed percentage were crucial for consistency, because health care providers (physicians, nurse practitioners, physician assistants) typically recorded 0.01 cm² for healed areas to ensure accurate measurement and ongoing patient follow-up. Patients continued follow-up at the discretion of their provider to ensure the wound or wounds remained closed until discharge from wound medicine services with a documented wound area of 0.0 cm2.
Design and procedures
This retrospective study focused on Medicare patients who underwent CTP treatment for chronic wounds. The primary aim was to assess the efficacy of CTP applications needed for wound closure and to analyze WAR rates in various chronic wounds. The study received private funding and did not accept vendor sponsorship or grants. Reimbursement for these CTP treatments followed local MAC guidelines throughout the study period. The current study was privately funded, and no vendor sponsorship or grants were accepted. Institutional review board approval for this retrospective study was obtained through Sterling IRB (ID number 12753).
Wound providers adhered to established institutional policies for CTP applications, ensuring comprehensive care. This included a mandatory 4-week period of SOC treatment, involving sharp and/or ultrasonic debridement before initiating CTP applications. Providers verified the absence of necrotic tissue or infection, assessed perfusion adequacy, and implemented proper off-loading or compression measures prior to beginning the CTP application series. Smoking cessation counseling was provided as needed. For patients with uncontrolled diabetes, interventions included diet guidance, medication adjustment, and encouragement of compliance, with referrals to primary care or endocrinology as necessary. Primary dressings were chosen to secure CTP applications, while secondary dressings managed wound drainage. Several off-loading techniques were used and were individualized to the patient and wound etiology. Off-loading wounds located on plantar surfaces included pressure relief through the use of total contact casts or other orthotic plantar relief shoes. VLU relief treatment included compression therapy for ambulatory venous pressure reduction. Tension relief measures were individualized to the patient’s wound and location. Secondary dressings were changed 2 or 3 times weekly based on wound drainage, while primary dressings typically remained in place for approximately 7 days. Patients underwent weekly follow-up with wound providers to repeat the CTP application process until wound closure or completion of up to 10 applications. This treatment adhered to MAC limitations outlined in the LCD policy. No patient exceeded 10 CTP applications, per policy.
Outcome measures
The primary outcomes assessed included the percentage WAR at each CTP application and the comparison of WAR from the start to the end of the CTP application series. Secondary outcomes encompassed the average wound size at each CTP application and the number of wounds that achieved healing upon completion of the CTP application series. Patient demographics were detailed for descriptive purposes within the sample studied. Comorbidity selection was based on a manual chart review to identify prevalent comorbidities often encountered in the private wound care setting. Mean (SD) hemoglobin A1c levels were reported for sample representation. Wound characteristics, such as initial wound depth structure before CTP application, were also documented. The specific CTPs used were described to characterize the type of CTP used.
Sample selection
A dataset was compiled by identifying Medicare-insured patients who underwent a CTP application between January 1, 2018, and December 31, 2023. Medicare insurance was the primary criterion for inclusion, because most patients receiving CTP therapy were eligible for up to 10 applications, aligning with the study objectives. ICD-10 diagnosis codes were used to identify patients with wound etiologies including DFU, VLU, trauma wounds, surgical wounds, and nonhealing chronic wounds. Patients receiving more than 10 CTP applications and those who did not complete their series within 16 weeks were excluded in order to standardize treatment protocols for all chronic wounds being evaluated. Additionally, patients with a greater than 21-day interval between CTP applications were excluded from the analysis. Patients whose wounds increased in size after the fourth CTP application were also excluded, following 2024 LCD policy guidelines at the time, which discouraged continued CTP applications if prior treatments were unsuccessful. Unsuccessful was defined as variables preventing progress to wound closure or wound improvement.15 Furthermore, patients diagnosed with connective tissue diseases were assessed to ascertain whether these conditions affected healing within cutaneous tissues (eg, Raynaud disease, calciphylaxis, pyoderma gangrenosum). Connective tissue diseases that affected the skin tissue were excluded from the review. Patients undergoing chemotherapy or radiation therapy at the time of CTP application, or who had previously received radiation at the wound site, were also excluded from the study due to radiation’s potential effect on cellular function impairment. The criterion of including wounds between 0 cm2 and 25 cm2 was consistent with most prior CTP studies in the reviewed literature.5,16-18 Additional exclusion criteria are listed in Figure 1.
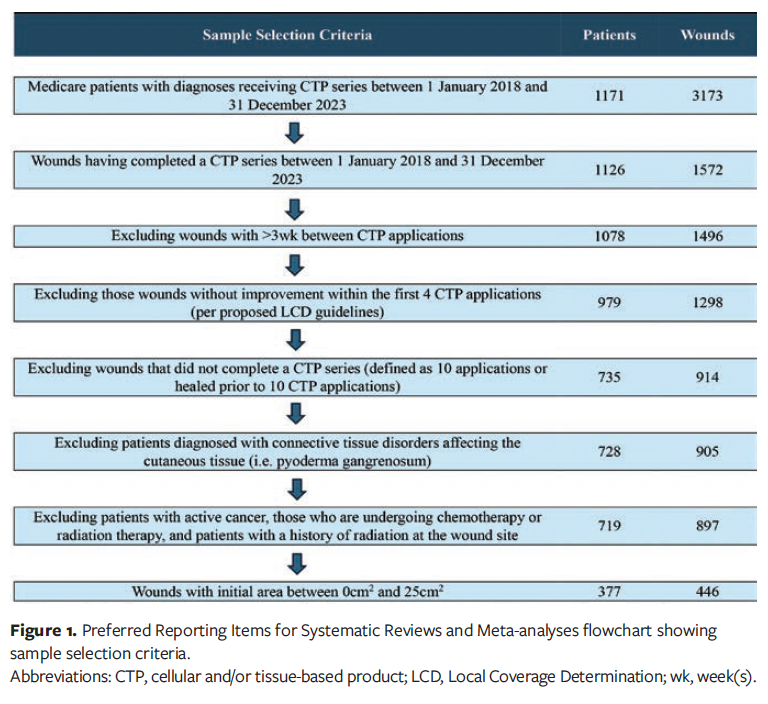
Analysis
Descriptive data encompassed the calculation of percentage WAR, which involved manual wound measurements taken before each CTP application and 1 week after completing the CTP application series. Microsoft Excel (Microsoft Corporation) was used for descriptive analysis, detailing patient demographics, baseline characteristics of patients and wounds, average wound size following each CTP application, average number of CTP applications administered, and percentage WAR achieved after each CTP application.
Inferential analyses involved comparing the initial wound area before starting the CTP series with the final wound area after completing the series. Statistical analysis was performed using SPSS version 29.0.2.0 (IBM Corporation) for all chronic wounds reviewed. A paired samples t test was used to compare means before and after the CTP application series, with effect sizes assessed using Cohen d. The Pearson correlation coefficient was used to examine correlations between wound sizes and the number of CTP applications. A 95% CI was used, with findings deemed statistically significant when the P value was less than .05. Outliers were identified and removed based on z scores, excluding percentage WAR falling outside 3 SD of the mean.
Patient, wound, and product characteristics
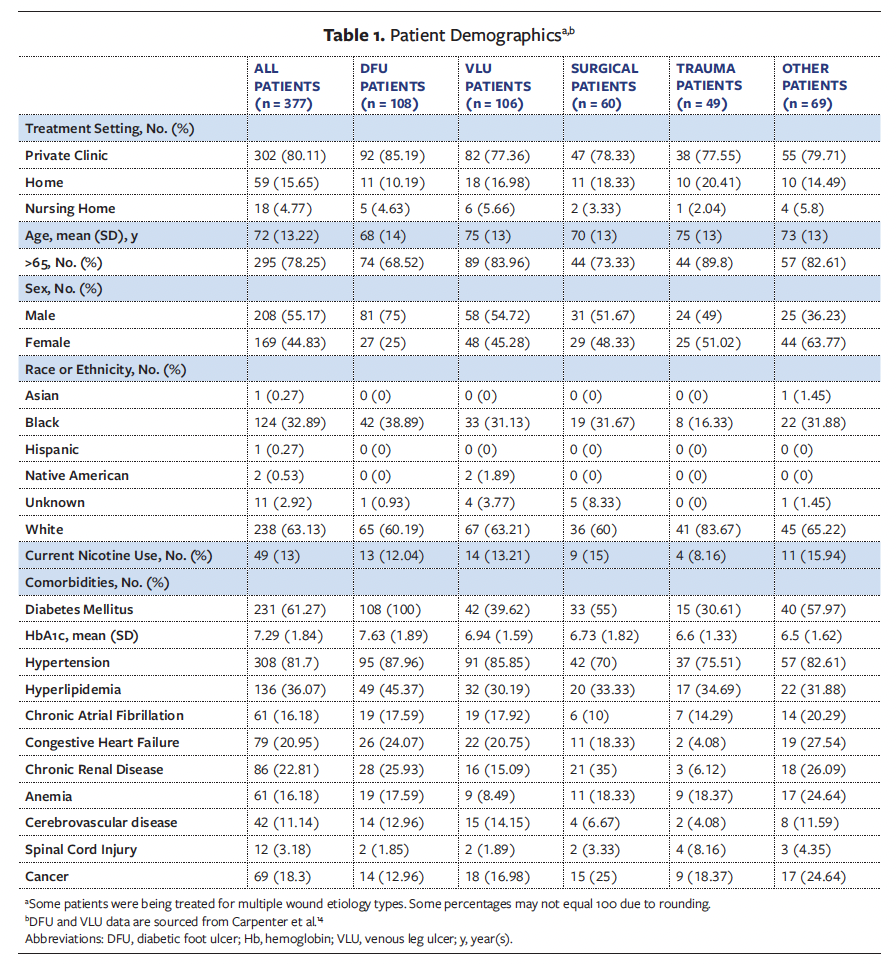
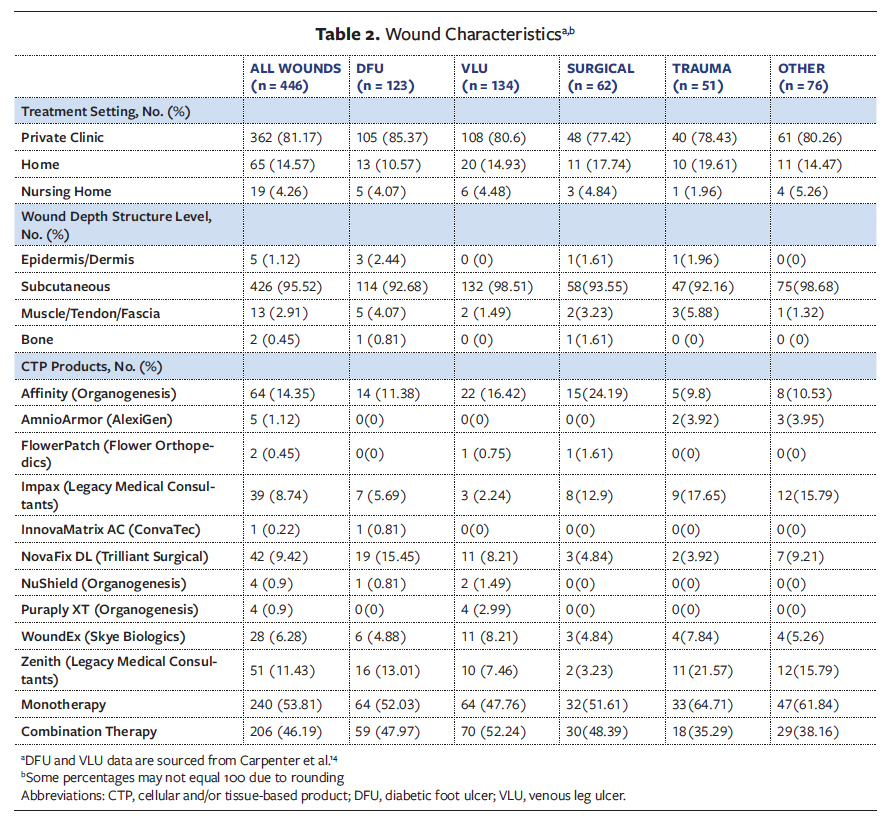
Patient demographics are detailed in Table 1. Patients were treated in the private practice wound clinic (80.11%), home (15.65%), and the nursing home settings (4.77%). The majority of patients included in this retrospective analysis were male (55.17%), and 78.25% of all patients were aged 65 years and older. The primary racial groups represented were Black (32.89%) and White (63.13%), and 13% of all patients were current smokers. Diabetes mellitus and hypertension were the predominant comorbidities among the reviewed sample. Table 2 outlines wound characteristics. The majority of wounds reviewed in this retrospective study were full-thickness wounds extending to the subcutaneous tissue (95.52%). Treatment of wounds with CTPs varied, with 53.81% receiving monotherapy and 46.19% receiving combination therapy.
Results
A total of 446 wounds were reviewed for analysis, of which 123 were DFUs, 134 were VLUs, 62 were surgical wounds, 51 were trauma wounds, and 76 were undefined “other” chronic wounds. Across diverse wound etiologies, the WAR demonstrated a consistent and progressive decline, as illustrated in Figure 2. Moreover, each subsequent CTP application yielded incremental improvements in average percentage WAR, with the trend evident across all chronic wound types, as shown in Figure 3.
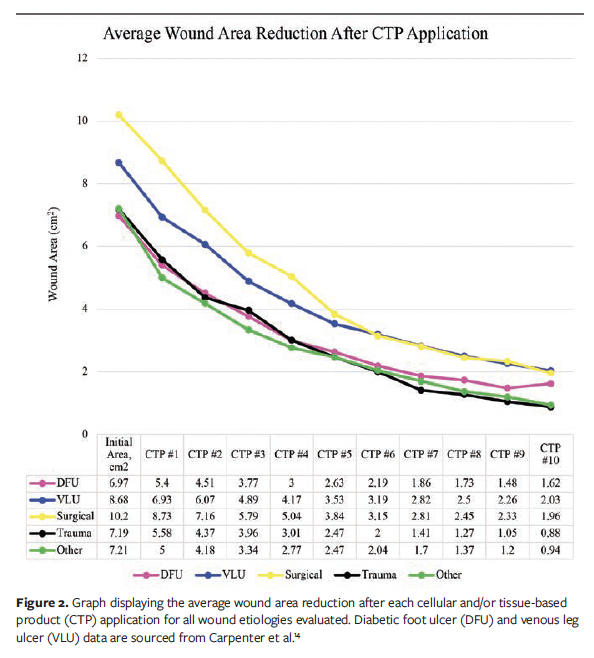
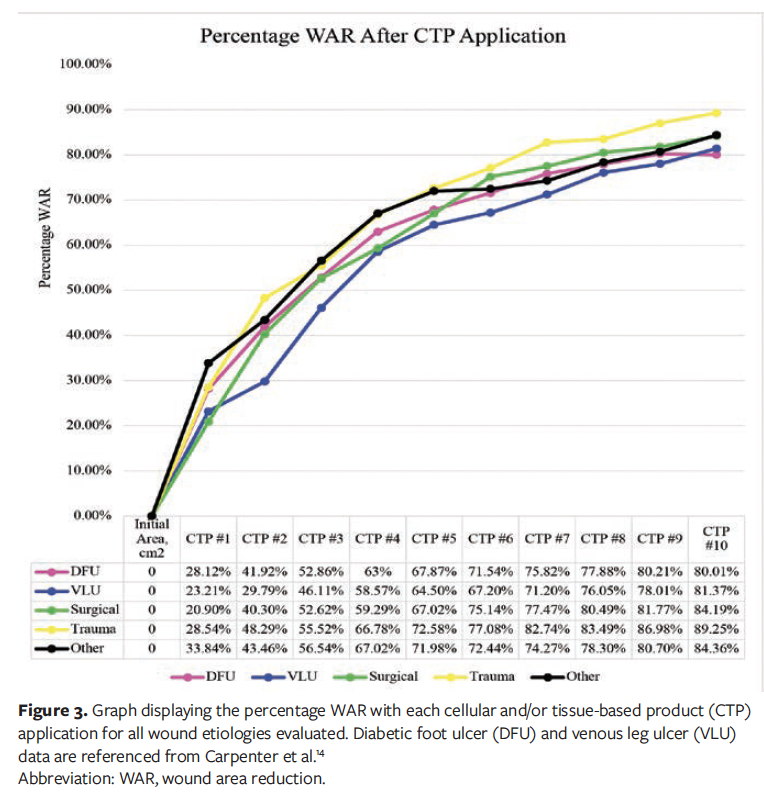
This study is based on previously established methodology, and (per figure legends and table footnotes) data on DFUs and VLUs were previously published in Carpenter et al.14 The DFU sample exhibited an average (SD) initial wound area of 6.97 (6.54) cm², and an average wound area of 1.62 (3.54) cm² after the final CTP application.14 The average (SD) number of CTP applications for the DFU sample was 8.4 (2.6).14
Similar trends were observed in VLUs during CTP treatment. The average (SD) initial wound area for the VLU sample was 8.68 (6.65) cm², and the average wound area after the final CTP application was 2.03 (4.3) cm².14 The VLU sample received an average (SD) of 7.63 (2.95) CTP applications.14
Surgical wounds were observed to have similar trends during CTP treatment. The average (SD) initial wound area for the surgical wound sample was 10.2 (6.55) cm2, and the average wound area after the final CTP application was 1.96 (3.4) cm2. The surgical wound sample was noted to have received an average (SD) of 7.8 (3.19) CTPs, with a median of 10.
The trauma wound sample exhibited an average initial wound area of 7.19 (6.86) cm2. The average wound area after the final CTP application was 0.88 (2.1) cm2.
Lastly, the other chronic wounds also had a progressive increase in percentage WAR with each CTP application. The average (SD) initial wound area for the other sample was 7.21 (6.3) cm2, and the average wound area after the final CTP application was 0.94 (2.38) cm2. The other chronic wound sample was noted to have received an average (SD) of 6.93 (3.21) CTPs, with a median of 8.
Healed wound characteristics
A total of 227 wounds achieved healing within a series of 10 CTP applications (50.9%). These 227 wounds comprised 50 DFUs (22.03%), 72 VLUs (31.72%), 27 surgical wounds (11.89%), 32 trauma wounds (14.1%), and 46 other chronic wounds (20.26%). The mean (SD) number of CTP applications for those DFUs noted healed was 6.06 (2.74), with a median number of 6. The mean (SD) number of CTP applications for healed VLUs was 5.57 (2.69), with a median number of 5. Healed surgical wounds required an average of 5.63 (3.13) CTP applications, with a median number of 5. Healed trauma wounds required an average of 4.38 (2.32) CTP applications, with a median number of 6. Lastly, the average number of CTP applications for other chronic wounds was 5.09 (2.71), with a median number of 5. Among the healed wounds, 146 (64.32%) received monotherapy CTP applications, while 81 (35.68%) received a combination of different CTPs throughout the series (Table 3). Additionally, characteristics of CTP monotherapy were analyzed. These findings are presented in Table 4, showing varied frequencies of healing among wounds treated with monotherapy CTP types.
Wound size and number of CTP applications
A paired samples t test was conducted for all chronic wounds to assess whether there was a clinically significant difference between initial wound area and final wound area following a CTP series. Additionally, Cohen d was also used to provide an accurate representation of the effect size for each wound etiology. Cohen d proposes that effect sizes can be classified as small (d = 0.2), medium (d = 0.5), and large (d = 0.8).19 This framework is widely adopted across wound medicine research domains.19
All wounds were noted to have a significant decrease in wound area after final CTP application compared with initial wound area, in addition to large effect sizes. The analysis of surgical, trauma, and other chronic wounds revealed consistent findings with comparative DFU and VLU literature.14 The surgical wounds analysis indicated a significant decrease from an initial mean (SD) area of 10.2 (6.55) cm2 to 1.96 (3.4) cm2 (t(61) = 10.848; P < .001), with a large effect size (Cohen d = 1.38). Trauma wounds were observed to have similar results, with a significant mean area reduction from 7.19 (6.86) cm2 to 0.88 (2.1) cm2 (t(50) = 6.862; P < .001), with a large effect size (Cohen d = 0.96). Lastly, other chronic wounds were also noted to have a significant mean area reduction from 7.21 (6.3) cm2 to 0.94 (2.38) cm2 (t(75) = 8.771; P < .001), with a large effect size (Cohen d = 1.0).
These findings align closely with the cohort published by Carpenter et al14 in 2024 examining DFUs and VLUs. Similarly, that study demonstrated statistically significant WAR for DFUs, having decreased from mean (SD) 6.97 (6.54) cm2 to 1.62 (3.54) cm2 (t(122) = 10.59; P < .001), with a large effect size (Cohen d = 0.96). Further, VLUs also revealed reduced mean wound area from 8.68 (6.65) cm2 to 2.03 (4.3) cm2 (t(133) = 12.97; P < .001), with a large effect size (Cohen d = 1.1).14 Across all wound etiologies, the consistent statistical significance and large effect sizes suggest a robust and promising therapeutic approach using CTP application. Detailed results of the paired samples t test are reported in Table 5, and Cohen d results are presented in Table 6.
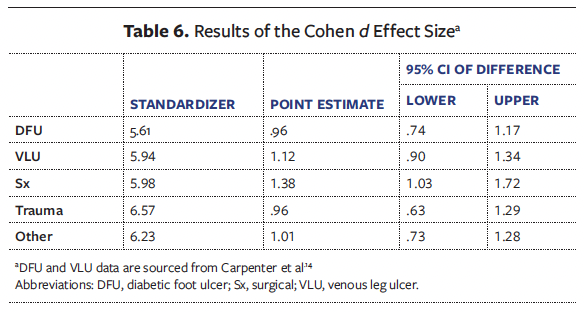
The clinical significance of these findings surpasses mere statistical analysis, offering a transformative approach to wound healing across various wound etiologies. The CTP intervention exhibited substantial healing potential, leading to a marked reduction in wound areas across diverse wound types. The consistency of these results across surgical, traumatic, and chronic wounds, coupled with their alignment with existing research on DFUs and VLUs, further reinforces the clinical relevance of these findings.
The Pearson correlation coefficient was used to examine the relationships between wound areas following each CTP application and the number of applications in the series (Table 7). The Pearson correlation coefficient measures the linear association between variables, ranging from −1 to +1, with values near +1 indicating strong positive relationships, values near −1 representing strong negative relationships, and values close to 0 suggesting minimal or no linear relationship.20
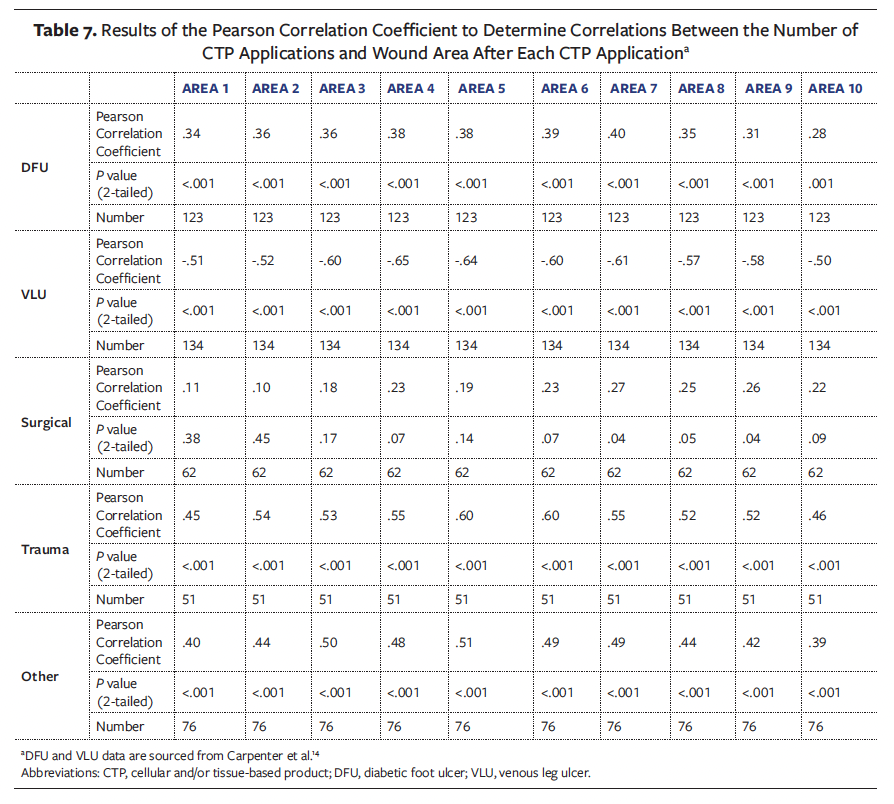
This retrospective analysis revealed varying correlation patterns between wound areas after each application and the number of CTPs applied. According to the Carpenter et al14 cohort, DFUs exhibited a weak positive correlation, with an initial correlation of r(123) = .34 (P < .001), and after 10 applications exhibiting a correlation of r(123) = .28 (P = .001). In contrast, VLUs demonstrated a more pronounced pattern, showing a moderate positive correlation initially of r(134) = .51 (P < .001) and a moderate negative correlation after 10 applications of r(134) = −.502 (P < .001).14
Surgical wounds displayed a non-statistically significant weak positive correlation, increasing from r(62) = .11 (P < .38) at the first application to r(62) = .22 (P = .09) after the 10th application. Similarly, trauma wounds showed a consistent weak positive correlation, ranging from r(51) = .45 (P < .001) initially to r(51) = .46 (P = < .001) after 10 applications. Other chronic wounds followed a comparable trend, with a weak positive correlation of r(76) = .4 (P < .001) at the initial application, slightly decreasing to r(76) = .39 (P = < .001) after the 10th application.
These correlation findings, when compared with the previous cohort literature examining DFUs and VLUs,14 highlight the nuanced relationship between wound areas after each application and the number of CTPs applied.
Discussion
This research focused on evaluating clinical outcomes regarding characteristics of healed wounds treated with CTP applications and the rate of WAR over the course of the CTP series. The primary objectives of this research were twofold. First, it aimed to assess whether the application of CTP could reduce wound size, and second, it sought to identify the optimal number of CTPs needed for effective wound reduction and closure. Significant reductions in average wound area were observed after each CTP application. Furthermore, statistically significant findings were also observed when comparing initial wound area averages with averages after completing the CTP application series, showing large effect sizes for all wound etiologies.
The findings of this retrospective analysis show CTP efficacy in treating surgical, trauma, and other chronic wounds comparable to that previously reported for chronic DFUs and VLUs. The healing rates observed in the current cohort study closely parallel those reported by Carpenter et al,14 suggesting that the therapeutic benefits of CTP extend beyond traditional applications in DFUs and VLUs. The 16-week WAR of 84.19% achieved in the surgical wound group, 89.25% achieved in the trauma wound group, and 84.36% achieved in the other chronic wound group aligns with the 80.01% closure rate reported for DFUs and the 81.37% closure rate for VLUs in the retrospective analysis by Carpenter et al.14 This consistency across different wound etiologies suggests that CTPs primarily address common underlying pathophysiological mechanisms that impair healing in chronic wounds, regardless of the initial cause. Notably, this cohort analysis also revealed similar findings regarding the number of CTP applications required to achieve the wound closure throughout all chronic wounds was approximately 8 applications. Further, it should be noted that the number of healed wounds within each etiology type was notably higher in surgical wounds (43.55%) and trauma wounds (62.75%), compared with DFUs (40.65%) and VLUs (53.73%).
The standard treatment for chronic wounds varies depending on the etiology of the wound. However, best practice includes ensuring wounds have a clean wound bed and are free of infection; this is often done through adequate debridement technique. Conventional therapy or SOC remains the primary approach for managing chronic wounds. However, this retrospective analysis supports incorporating CTP applications alongside conventional therapies to increase the likelihood of reducing wound area and achieving complete wound closure for chronic wounds. Despite the widespread use of negative pressure wound therapy for postoperative wounds, there is a lack of rigorous comparative research against established SOCs. While this treatment is commonly applied to many surgical wounds and involves significant costs, the overall efficacy remains uncertain. In reality, there is insufficient evidence to substantiate a standardized approach for managing these diverse wound types.
Careful inclusion selection processes were set forth to enhance the validity of the study and support the use of CTP applications in treating chronic wounds. Patients who did not meet the inclusion criteria were systematically excluded to maintain the integrity and focus of the research. This study excluded patients with advanced systemic conditions (eg, severe pyoderma gangrenosum) and those undergoing active cancer chemotherapy, because these factors could potentially distort the results or compromise the accurate assessment of the CTPs’ effectiveness. Additionally, patients with private insurance are often approved for more CTP applications than the limit imposed for this study; thus, these patients could not be compared with the majority of patients approved to have 10 CTP applications per LCD guidelines established by local MACs. By excluding these patients, the study aimed to create a more homogeneous participant group, ensuring that the observed effects on wound healing could be more confidently attributed to CTP therapy rather than extraneous variables. This careful selection process enhances the validity of the findings and supports the development of more effective treatment strategies for chronic wounds. It could be argued that patients with these underlying conditions should not receive CTPs at all. However, numerous case reports have documented patients, particularly those with pyoderma gangrenosum, experiencing benefits from multiple applications of CTPs. Consequently, the study’s exclusion criteria are not intended to discourage future therapeutic options for these patients; rather, they are intended to ensure a more standardized and reliable dataset.
In addition, this study represents a unique look at CTP application in the community setting. It should be noted that such application significantly reduces the cost of transportation to and from a medical center. While home-based application may potentially compromise therapeutic effectiveness due to limitations in wound debridement and preparation, it simultaneously expands treatment accessibility to underserved communities and broadens the demographic reach of patient care.
Regulatory bodies play a significant role in determining the frequency of CTP applications for treating chronic wounds. It is essential to base guidelines on substantial evidence to ensure appropriate use of CTP applications. Evidence supports the use of CTP applications to effectively reduce chronic wound sizes.3,5,8-11 The current study specifically examines wound area changes with each CTP application over a 16-week period in treating chronic wounds of various etiologies. The study observed average reductions in wound size per CTP application, with a maximum of 10 applications noted. Ongoing evidence-based research can provide further insights into the optimal number of CTP applications required for effective chronic wound size reduction for wounds other than the commonly treated DFUs and VLUs. Future studies are needed to validate the promising outcomes of these treatments with robust methodologies and larger sample sizes to enhance care for patients with challenging wounds. Ongoing evidence is necessary to ensure adequate inclusion of CTPs in regulatory guidelines of various chronic wounds.
Limitations
Several limitations were observed in the data analyzed. Enhancing the impact and statistical power of the analysis would require a larger sample size. Moreover, the retrospective analysis was strictly confined to Medicare patients in private practice, nursing home, and home health settings, thus significantly limiting the generalizability of the findings. Access to hospital outpatient department (HOPD) data was severely restricted, precluding comprehensive analysis across all care settings. This limitation is particularly notable given that most chronic wound care clinics operate within HOPD settings; that is, the majority of patients with chronic wounds are typically treated in these environments. By excluding HOPD settings, the study’s sample may introduce substantial selection bias, potentially skewing the results and markedly reducing the applicability to the broader wound care population. Consequently, the sample was predominantly composed of patients aged 65 years or older, further narrowing the representativeness of the findings. Future studies should consider including patients with alternative insurance providers to broaden the age range and increase the sample size, thereby enabling more generalizable conclusions regarding the efficacy of CTP therapy for chronic wound treatment.
This study had data collection limitations as well. Automated reports were used to review all charts, and this was supplemented by a manual review of individual charts. The accuracy of medical coding was crucial for identifying the sample analyzed. Another limitation was the focus on wound sizes between 0 cm² and 25 cm² for inclusion in the study. Future research may be necessary to assess the effectiveness of CTPs for wounds larger than 25 cm², and to explore alternative advanced therapies to enhance wound healing rates. Further, exploring treatment with CTPs in chronic wounds with advanced depths would also provide insight into the effectiveness of CTP treatment on exposed bone and tendon within wound beds.
A critical limitation of this study is the absence of a control arm demonstrating WAR without CTP application. The natural progression of such wounds remains poorly understood, highlighting the need for a comprehensive repository documenting their intrinsic healing characteristics. While this study concentrates on CTP efficacy and application, it would significantly benefit from a comparative control group. Future clinical care models may potentially address this methodological gap by developing an appropriate comparator.
The variable time to heal was not applicable to all subjects in this review, because healing was not observed in all patients upon completion of the series of CTP applications. Standardized reporting protocols are essential to obtain more precise time frames and outcomes. Given the study’s focus, wound sizes were evaluated only for a maximum of 16 weeks. Incorporating the variable of time to heal could also offer insights into the predictability of outcomes following the completion of CTP applications for chronic wounds.
The study faced substantial limitations regarding the characteristics of healed wounds in relation to the type of CTPs applied. Among chronic wounds that healed during the CTP application series, there was considerable variation in the types of CTPs used, with the vast majority being placental-based products. Given that most of the sample received multiple CTPs throughout the series, the data do not support favoring one specific CTP over others. Further research is needed to compare the efficacy of different US Food and Drug Administration–approved CTPs available for treating chronic wounds. Additionally, research comparing the effectiveness of CTP monotherapy is essential to inform evidence-based decisions regarding the number of applications required.
Future studies should carefully consider and thoroughly report appropriate outcome analyses to reduce risk of bias and improve the quality of evidence. Additionally, research should investigate the cost-effectiveness of using specific CTPs to reduce wound sizes in chronic wounds. The comparable efficacy of CTPs across different chronic wound types suggests that wound care protocols could potentially be standardized, thus simplifying clinical decision-making. However, cost-effectiveness analyses specific to surgical and trauma wounds are needed, because the economic considerations may differ from those established for DFUs and VLUs. Evaluating cost issues is crucial, because they frequently act as barriers preventing the adoption of new treatments over established commercial standards. Subsequent evaluations should address these cost considerations and other relevant factors to facilitate the acceptance of new therapies.
Conclusion
Managing chronic wounds poses a considerable health care challenge worldwide, necessitating comprehensive strategies to ease patient suffering and decrease chronic wound morbidity and mortality. The findings of this retrospective analysis show CTP efficacy in treating surgical, trauma, and other chronic wounds comparable to that previously reported for chronic DFUs and VLUs. These findings could inform recommendations regarding the number of CTP applications needed for effective treatment of chronic wounds other than DFUs and VLUs. Lastly, this retrospective review suggests that the CTP approach is not a singular success; however, it is a potentially universal therapeutic strategy with broad applicability. Further research should focus on identifying specific patient variables to optimize outcomes with CTP therapy across all chronic wound etiologies. Additionally, future randomized controlled trials should investigate CTP treatment protocols for chronic surgical and trauma wounds.
Author & Publication Information
Authors: Shaun Carpenter, MD, CWSP; Angelina Ferguson, DNP, FNP, CWS; Devinna Bahadur, DNP, MSN Ed, AGACNP-BC; Amanda Estapa, ACNP-BC, CWS; Jamie Bahm, RHIA, CCS; and Sadie Burst, BS
Affiliation: MedCentris Wound Healing Institute; Hammond, LA, USA
Disclosure: The authors disclose no financial or other conflicts of interest.
Ethical Approval: Institutional review board approval for this retrospective study was obtained through Sterling IRB (ID number 12753).
Correspondence: Angelina Ferguson, DNP, FNP, CWS; Attn: Dr. Angelina Ferguson, 16065 Lamonte Drive, Hammond, LA 70403; Angelina.ferguson@medcentris.com
Manuscript Accepted: May 19, 2025
References
1. Sen CK. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care (New Rochelle). 2019;8(2):39-48. doi:10.1089/wound.2019.0946
2. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27-32. doi:10.1016/j.jval.2017.07.007
3. Svobodova A, Horvath V, Balogh L, et al. Outcome of application of cryopreserved amniotic membrane grafts in the treatment of chronic nonhealing wounds of different origins in polymorbid patients: a prospective multicenter study. Bioengineering (Basel). 2023;10(8):900. doi:10.3390/bioengineering10080900
4. Vecin NM, Kirsner RS. Skin substitutes as treatment for chronic wounds: current and future directions. Front Med (Lausanne). 2023;10:1154567. doi:10.3389/fmed.2023.1154567
5. Norman G, Westby MJ, Rithalia AD, Stubbs N, Soares MO, Dumville JC. Dressings and topical agents for treating venous leg ulcers. Cochrane Database Syst Rev. 2018;2018(6):CD012583. doi:10.1002/14651858.CD012583.pub2
6. Ontario Health (Quality). Skin substitutes for adults with diabetic foot ulcers and venous leg ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2021;21(7):1-165.
7. Mohammed YA, Farouk HK, Gbreel MI, et al.
Human amniotic membrane products for patients with diabetic foot ulcers. do they help? a systematic review and meta-analysis. J Foot Ankle Res. 2022;15(1):71. doi:10.1186/s13047-022-00575-y
8. Probst S, Saini C, Gschwind G, et al. Prevalence and incidence of venous leg ulcers-a systematic review and meta-analysis. Int Wound J. 2023;20(9):3906-3921. doi:10.1111/iwj.14272
9. O’Meara S, Al-Kurdi D, Ologun Y, Ovington LG, Martyn-St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev. 2014;2014(1):CD003557. doi:10.1002/14651858.CD003557.pub5
10. Bay C, Chizmar Z, Reece EM, et al. Comparison of skin substitutes for acute and chronic wound management. Semin Plast Surg. 2021;35(3):171-180. doi:10.1055/s-0041-1731463
11. Wardhana A, Valeria M. Efficacy of skin substitutes for management of acute burn cases: a systematic review. Ann Burns Fire Disasters. 2022;35(3):227–236.
12. Tettelbach W, Cazzell S, Reyzelman AM, Sigal F, Caporusso JM, Agnew PS. A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dHACM allograft in the management of diabetic foot ulcers: a prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics. Int Wound J. 2019;16(1):19-29. doi:10.1111/iwj.12976
13. Caputo WJ, Vaquero C, Monterosa A, et al. A retrospective study of cryopreserved umbilical cord as an adjunctive therapy to promote the healing of chronic, complex foot ulcers with underlying osteomyelitis. Wound Repair Regen. 2016;24(5):885-893. doi:10.1111/wrr.12456
14. Carpenter S, Ferguson A, Bahadur D, Estapa A, Bahm J. Evaluating the number of cellular and/or tissue-based product applications required to treat diabetic foot ulcers and venous leg ulcers in non-hospital outpatient department settings. Wounds. 2024;26(8):245-254. doi:10.25270/wnds/24092
15. Centers for Medicare and Medicaid Services. Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers. Medicare Coverage Database. Accessed May 5, 2024. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=39870&ver=4
16. Armstrong DG, Tettelbach WH, Chang TJ, et al. Observed impact of skin substitutes in lower extremity diabetic ulcers: lessons from the Medicare Database (2015-2018). J Wound Care. 2021;30(Sup7):S5-S16. doi:10.12968/jowc.2021.30.Sup7.S5
17. Chandler LA, Alvarez OM, Blume PA, et al. Wound conforming matrix containing purified homogenate of dermal collagen promotes healing of diabetic neuropathic foot ulcers: comparative analysis versus standard of care. Adv Wound Care (New Rochelle). 2020;9(2):61-67. doi:10.1089/wound.2019.1024
18. Lakmal K, Basnayake O, Hettiarachchi D. Systematic review on the rational use of amniotic membrane allografts in diabetic foot ulcer treatment. BMC Surg. 2021;21(1):87. doi:10.1186/s12893-021-01084-8
19. Légaré S, Chagnon M, Palijan A, Kojok K, Bissonnette R. Sensitivity of clinician-assessed efficacy outcome measurement instruments in trials of topical therapies for atopic dermatitis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2022;36(2):196-212. doi:10.1111/jdv.17743
20. Brydges CR. Effect size guidelines, sample size calculations, and statistical power in gerontology. Innov Aging. 2019;3(4):igz036. doi:10.1093/geroni/igz036
















