Effect of Social Determinants of Health on Clinical Trials Conducted for Diabetic Foot Ulcer and Venous Leg Ulcer at a Safety Net Hospital
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. Failure to adhere to study protocol is among the most common observations during inspections conducted by competent authorities for clinical trials. Objective. To evaluate patterns of protocol deviations, and to analyze how social determinants of health (SDoH) correlate with the rate of protocol deviations as indicators of study protocol noncompliance. Materials and Methods. Data obtained at a single clinical site from 19 clinical trials with a total of 186 subjects enrolled were analyzed retrospectively, and correlations between SDoH (eg, race/ethnicity, gender [eg, male/female], socioeconomic status, distance traveled, etc) and study protocol noncompliance (ie, rate of deviations) were examined. The Kruskal-Wallis test was performed to compare SDoH variables with rate of deviations per subjects enrolled. Associations between SDoH and deviations were examined using the Spearman correlation test. Results. A retrospective analysis showed that the majority of deviations were attributed to study visits that had not been performed in a timely manner or were missed, and study procedures that either were not performed or were completed late. The tests demonstrated no statistical significance between age, gender, and race and rates of dropout from the study (P = .1857, P = .3836, and P = .2150, respectively). Increased body mass index was associated with higher dropout rates (P = .0340), which can be an indicator of higher disease burden and an obstacle to trial participation. Conclusion. Further studies are warranted to investigate how quality in wound care clinical trials can be improved with identification of patients who need more resources based on their SDoH to efficiently mitigate risks, increase access to trials for disadvantaged populations, and improve study protocol compliance.
Abbreviations: BMI, body mass index; DFU, diabetic foot ulcer; IRB, institutional review board; SDoH, social determinants of health; VLU, venous leg ulcer.
According to Chan B et al in the year of 2017, the burden of DFUs and VLUs on the population heavily contributes to the total midrange estimates for all Medicare spending on wounds at $37.1 billion.1 This large financial burden has motivated increased research and development in the wound care field, as well as an increasing number of clinical trials in the past decade conducted with the goal of finding effective and lower-cost products for the treatment of chronic wounds.2
Institutions conducting clinical research are responsible not only for adherence to trial protocol but ultimately the healing of these chronic wounds. An increasing number of trials complicates issues with study protocol and regulatory compliance, which can further delay wound healing, because some trial results may not reach statistical significance due to issues with protocol compliance (eg, high rate of protocol deviations, incomplete data, high rate of dropouts, lack of inclusion of diverse populations as expected by regulatory authorities).3,4
Failure to comply with study protocol and procedures is one of the most common observations during inspections conducted by competent authorities for clinical trials. According to the US Food and Drug Administration Bioresearch Monitoring (BIMO) program inspections of investigative site metrics, approximately 30% of all warning letters were issued because investigators were failing to follow the investigational plan and study protocol.4 The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice E6(R3) and E8(R1) regulatory guidelines for conduct of clinical trials were recently revised, and regulatory authorities expect all stakeholders to adopt quality by design and risk-based quality management principles for clinical trials in order to proactively mitigate risks, improve quality of research conducted, decrease complexities, and engage participants with different backgrounds.5-7
Historically in pursuit of improving health care, there have been times in which clinical research was intertwined with not only racial exploitation but exploitation of those who may be hindered by socioeconomic constraints.8-10 To properly address these issues and conduct safe, efficient studies, clinical research institutes should be conducting analysis of protocol adherence as it relates to the key SDoH. Dedicated research teams can identify gaps in protocol adherence and provide support in more targeted ways.
Currently, it is also an expectation of regulatory authorities such as Food and Drug Administration (FDA) under Omnibus Reform Act of 2022 (FDORA) to engage with study participants up front, provide patient-centric solutions in the design of study protocol with the goal to diversify study populations, decrease burden to participation and improve access to innovative treatment options to a wider range of participants, and produce clinical trials outcomes that will mitigate regulatory risks. These risks include potential failure to approve submissions due to issues with generalizability of findings (ie, studied population was limited to specific characteristics and variability of response was not addressed in different race/ethnic groups, genders, ages, etc), leading to additional requirements and/or commitments for additional studies, limitations in prescribing information, and negative impact on post-marketing activities. Proactively addressing scientific gaps in clinical trials can help to avoid clinical and regulatory risks related to full characterization of the safety and efficacy of biomedical products.11
The purpose of this study was to evaluate patterns of protocol deviations and analyze how SDoH correlate with the rate of protocol deviations as indicators of study protocol noncompliance.
Materials and Methods
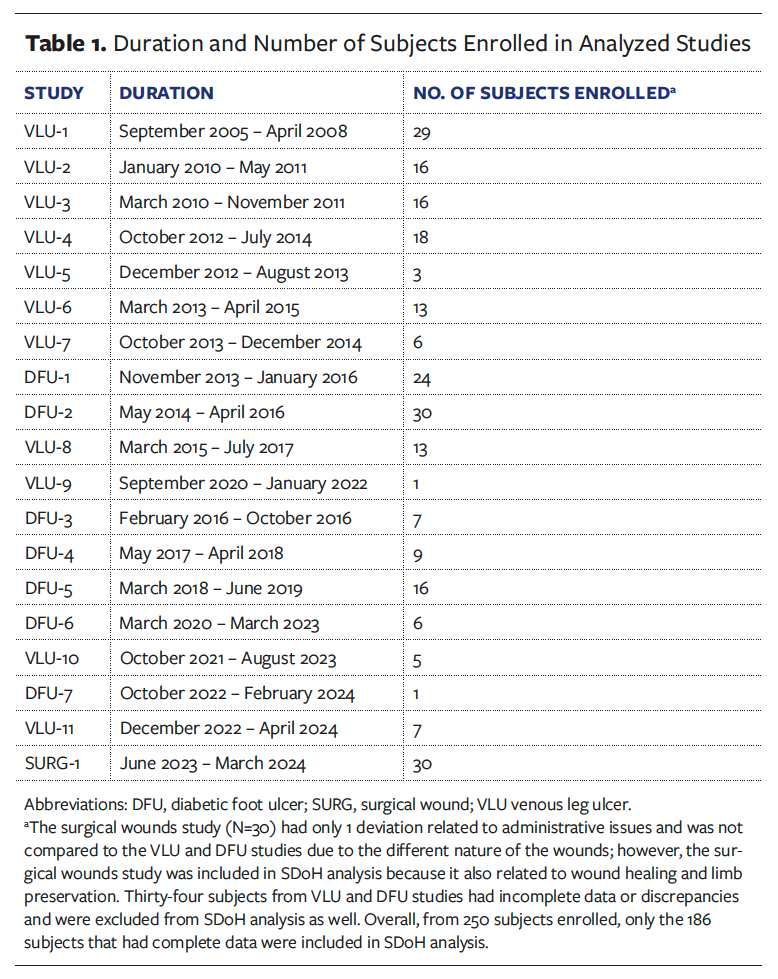
The authors retrospectively analyzed 19 clinical trials with a total of 186 subjects enrolled; these trials were conducted at an academically affiliated, tertiary, urban safety net hospital as a single clinical site. The research team on the department level consisted of 3 research coordinators and a research director, all full-time employees. IRB approval was obtained prior to conduct of any research activities. These trials had similar study designs and procedures, and involved products used in an effort to heal DFUs and VLUs. The objective was to compare rates of deviations for these 2 indications and examine how deviations, as indicators of study protocol nonadherence, relate to various SDoH that were selected by the World Health Organization (eg, income, education, housing security, access to health care services).12 The surgical wounds study had only 1 deviation related to administrative issues and was not compared to the VLU and DFU studies due to the different nature of the wounds; however, the surgical wounds study was included in SDoH analysis because it also related to wound healing and limb preservation.
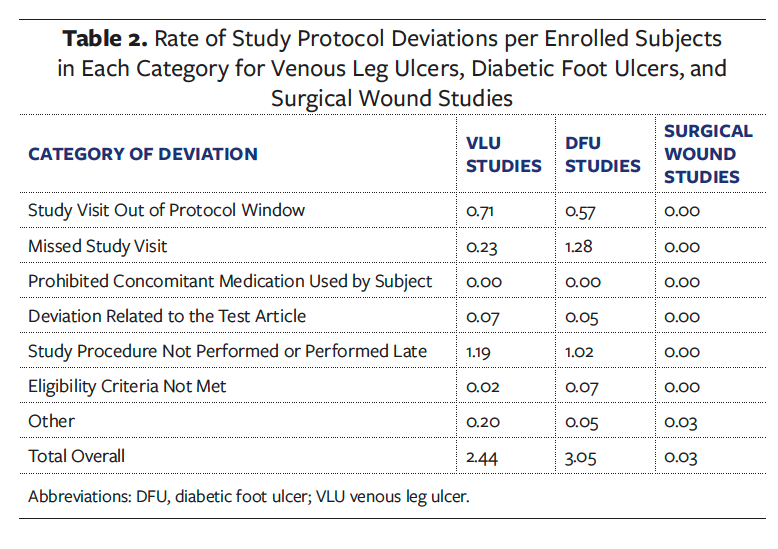
Study protocol deviations in each study were tracked by specific categories and ranked by severity and incidence rate, which was calculated by dividing the number of deviations by the number of subjects enrolled (Table 1), to determine root cause of deviation. Additionally, demographics were collected to indicate certain factors related to SDoH. The authors of the present study also conducted an analysis of the type of deviations that occurred based on specific study protocol (Table 2). Once informed consent was obtained, the patient was considered enrolled in the study and data collection had begun.
 Deviations were used to track study protocol and procedure noncompliance, as well as issues with adherence to investigational product regimens that may be affected by SDoH. The following SDoH variables were collected from electronic medical charts and assessed in correlation with study protocol deviations: race, gender, age, primary language, distance traveled to the clinical site, median household income by zip code, type of health insurance, and BMI. Income was calculated by zip code associated with patients at the start of trials, and census data from 2022 were used to determine median household income of that area.13 Distance traveled calculations were made by using the subject’s street address and calculating the distance from their home to the clinical site address where the study was conducted, as a consistent destination on the medical campus.
Deviations were used to track study protocol and procedure noncompliance, as well as issues with adherence to investigational product regimens that may be affected by SDoH. The following SDoH variables were collected from electronic medical charts and assessed in correlation with study protocol deviations: race, gender, age, primary language, distance traveled to the clinical site, median household income by zip code, type of health insurance, and BMI. Income was calculated by zip code associated with patients at the start of trials, and census data from 2022 were used to determine median household income of that area.13 Distance traveled calculations were made by using the subject’s street address and calculating the distance from their home to the clinical site address where the study was conducted, as a consistent destination on the medical campus.
All analyzed trials had a patient stipend, with an approximately similar amount per each completed study visit. All stipend amounts/payments were approved by Institutional Review Board and Clinical Trials Office as the organizational authorities, and these monies were never paid up front to any of the research subjects, in order to avoid the issue of the monies being an incentive to participate in studies. The payments were always made only after completion of all study protocol- required procedures and/or assessments for each study visit, and they had no effect on study participation or study protocol deviations.
Insurance was broken down into 3 categories based on the type. Individuals who carried a government- or state-serviced insurance plan were categorized as having “government insurance,” those with another form of insurance were categorized as having “private insurance coverage,” and those without insurance were categorized as “underinsured or uninsured.”
The data obtained from the 19 clinical trials were analyzed using SAS software (version 9.4; SAS Institute Incorporated). Descriptive statistics were used to categorize deviations. The distribution of Wilcoxon scores was examined, and Kruskal-Wallis tests were performed on data gathered to compare specific SDoH with rate of deviations per subjects enrolled. Associations between SDoH and deviations were examined using the Spearman correlation test.14 This statistical test was used to measure a linear correlation. If the Spearman coefficient (r) was between −1 and +1, that measured the strength and also direction of the association between 2 variables.14 If r equaled 0, then there was no relationship between examined variables and no correlation observed. If r was between 0 and 1, then there was a positive correlation observed: if 1 variable changed, then the other variable changed in the same direction. If r was between 0 and −1, then the correlation was negative; that is, when 1 variable changed, the other variable changed in the opposite direction. Specifically, when coefficient r was greater than 0.5, strong positive correlation between assessed variables was observed; when r was between 0.3 and 0.5 the correlation was moderate; and when r was between 0 and 0.3 a positive correlation was observed.15 The level of significance was set to .05, with a confidence interval of 95%.
Results
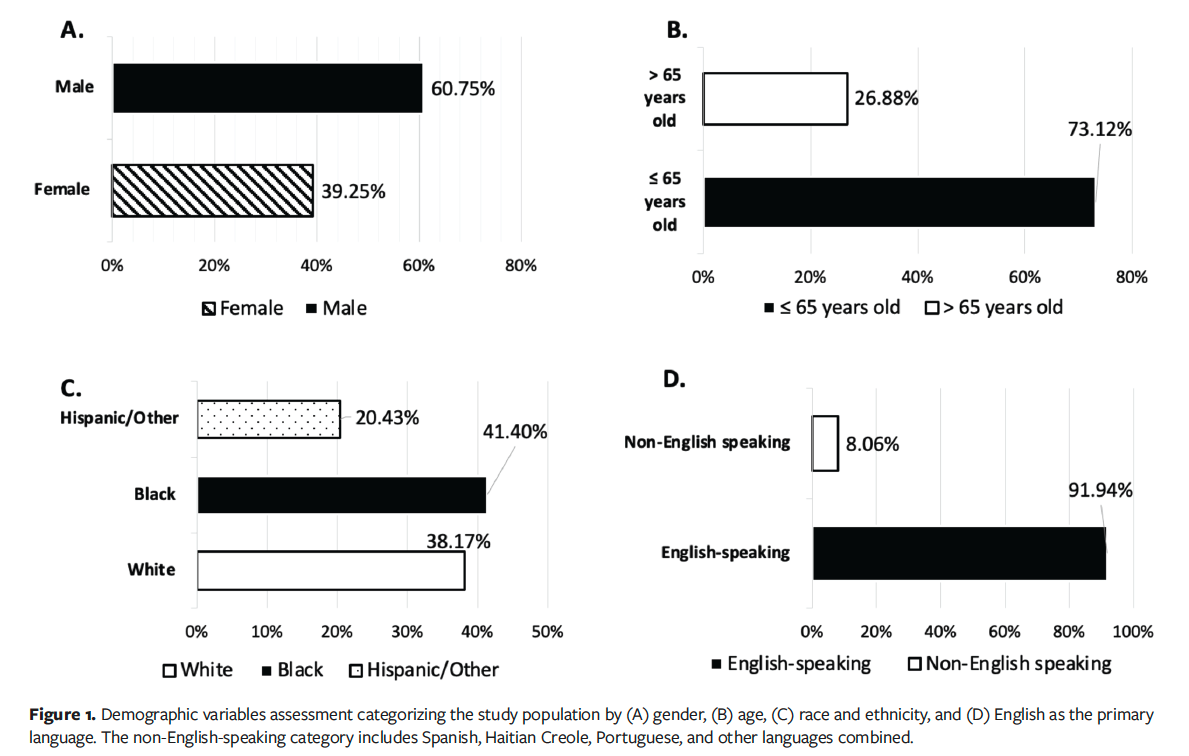
The results of the analysis from the 19 clinical trials, which were conducted with various products indicated for the treatment of VLUs and DFUs, are presented in Figure 1. Enrolled subjects who met eligibility criteria and who received at least 1 application of an investigational product were included in the analysis (n = 186). The analyzed population consisted of 60.75% males and 39.25% females (n = 113 and n = 73, respectively), with 73.12% of subjects older than age 65 years (n = 136), and subjects being predominantly English speaking (91.94% [n = 171]) (Figure 1). The included study populations were diverse, with 41.40% Black, 38.17% White, and 20.43% Hispanic or other ethnicities (Figure 1).
Comparative analysis showed that the overall rate of deviations per subject enrolled was 2.4 for the VLU studies and 3.0 for the DFU studies, as shown in Figure 2. The highest rate of deviations observed for both VLU and DFU studies was due to study procedure not performed or performed late (Table 2).
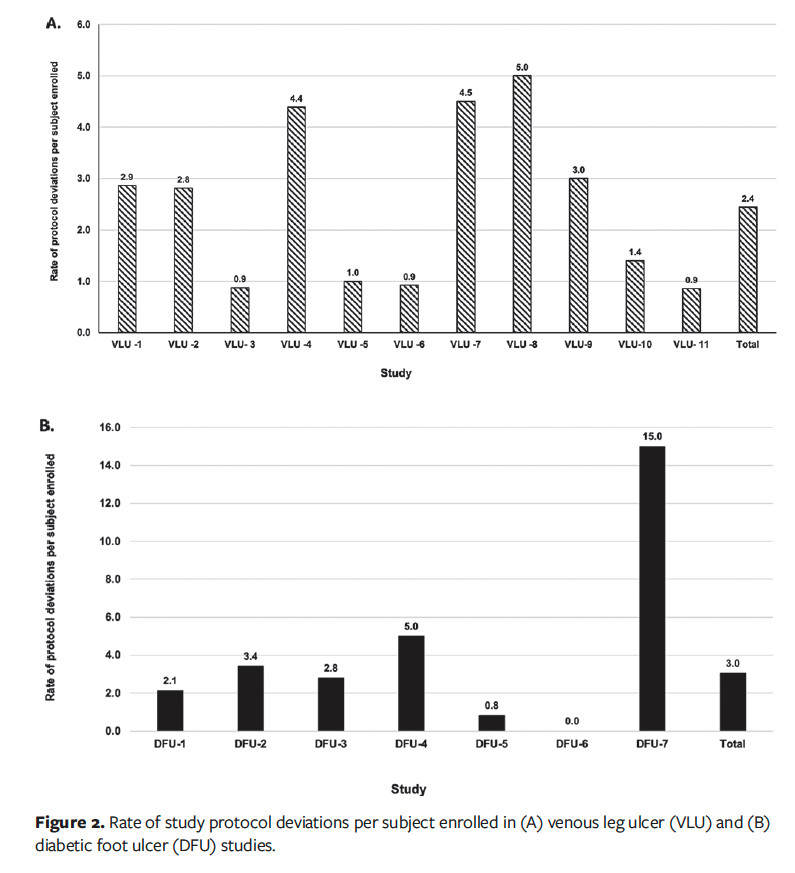
The findings of the present study revealed that some of the deviations were repetitive and not corrected in a timely manner in earlier conducted studies. For instance, 32 of the 83 deviations in the VLU-1 study (38.55%) were attributed to the “study procedure not performed or performed late” category, which were mainly related to a technical issue with the camera used to capture images to determine wound size and assess healing rate as a primary outcome measure for efficacy.
This issue in turn contributed to the overall higher rate of deviations per subject enrolled in the VLU-1 study, and in the later-conducted VLU-7 and VLU-8 studies, as shown in Figure 2A. Early identification of issues and performance of root cause analysis can help to prevent recurrence, reduce the number of deviations, and improve data quality and integrity.
As shown in Table 2, the rate of deviations was higher for VLU studies than for DFU studies for the category “study visit out of protocol window” (0.71 and 0.57, respectively), as well as for the category “study procedure not performed or performed late” (1.19 and 1.02, respectively). Remarkably, in the DFU studies subjects were more prone to miss study visits entirely, with a rate of deviations of 1.28 in this category compared with 0.23 for VLU studies (Table 2).
In summary, a retrospective analysis of deviations showed that the majority of study protocol nonadherence issues were attributed to required study visits not being performed in a timely manner or being missed, and to study procedures not performed or performed late. These results were similar between the 2 examined indications, as shown in Table 2.
More detailed examination of the category “study procedure not performed or performed late” revealed that protocol deviations were mostly related to inaccurate wound image capture, lack of adequate investigational drug or device services, issues with laboratory testing, and protocol-required ancillary assessments (eg, administration of quality-of-life questionnaires).
Kruskal-Wallis testing showed no statistically significant correlations observed between either gender (P = .0595) or different races (P = .0573), and number of deviations, as shown in Figure 3.
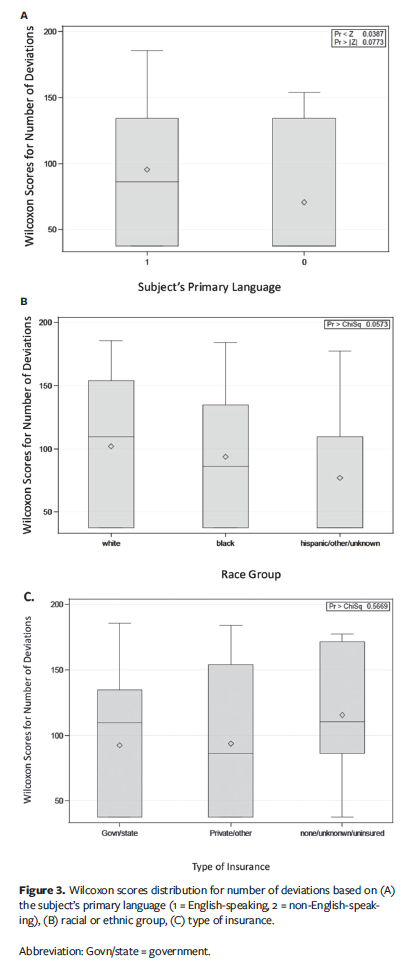
Language barrier is a major concern in health care. In examined clinical trials, no statistically significant correlations were detected between rate of deviations and subjects speaking a language other than English (P = .0773) (Figure 3A). Although these results were not statistically significant, a trend was noted that language barrier cannot be ruled out as a contributing factor for protocol deviations for non-English-speaking subjects as compared to English speakers.
Additionally, the present study assessed the rate of dropouts related to type of insurance and whether that had any bearing on protocol deviations. A Kruskal-Wallis test determined there was no significant difference by insurance type and effect of protocol deviations observed (P = .5669) (Figure 3C).
The Kruskal-Wallis and chi-square tests demonstrated no significant difference in study dropout rates by age (Figure 4A), gender, or race (P = .1857, P = .3836, and P = .2150, respectively).
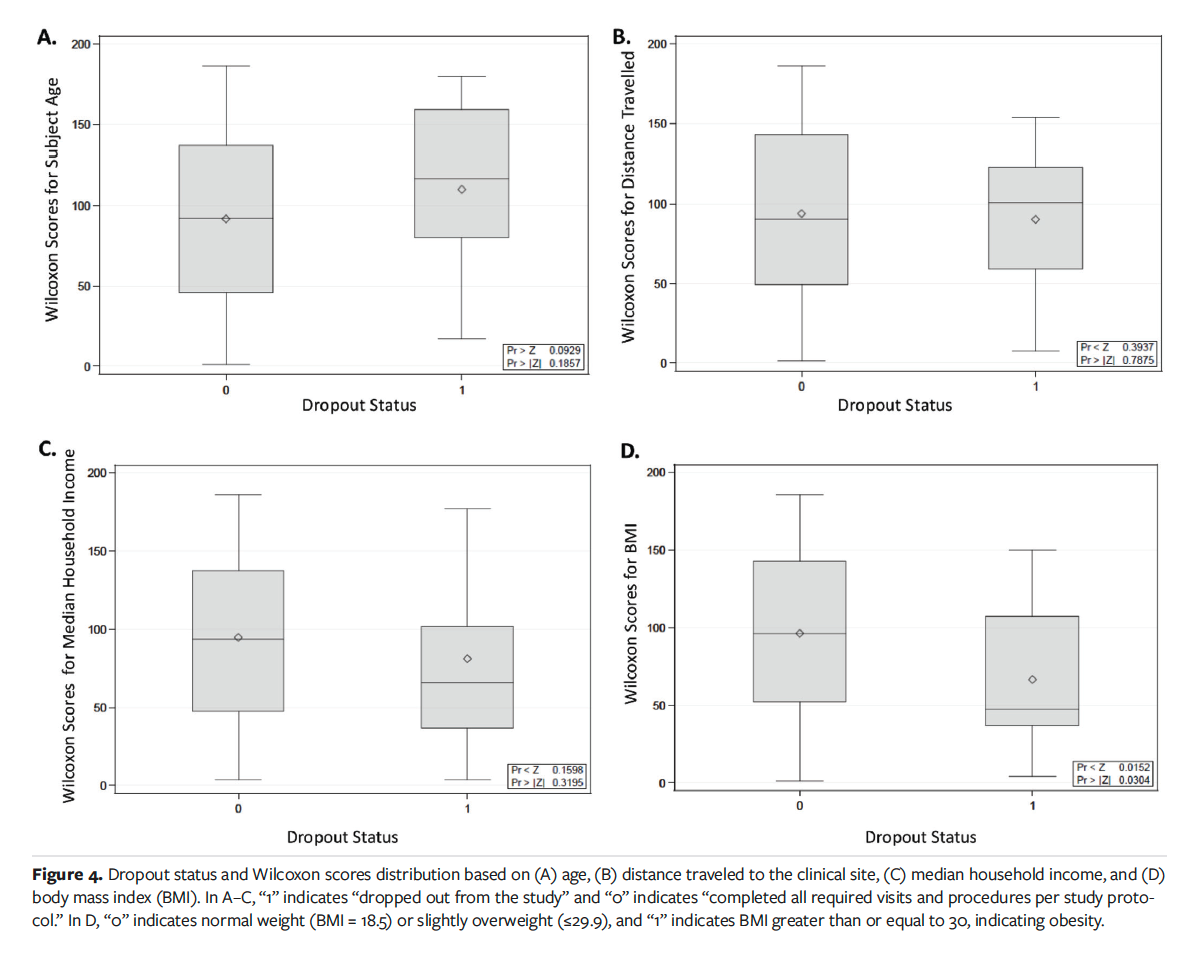
There is a burden associated with going to the hospital in terms of distance traveled to the clinical research site, time taken away from work, and financial considerations (eg, costs associated with gasoline needed to travel, cost of public transportation, and parking fees). The median household income was assessed with rate of protocol deviations and dropout rate. A Spearman correlation test determined no statistically significant findings with household income as it relates to number of deviations (P = .0675). However, the trend was noted that subjects with a lower household income tend to have more deviations than subjects with a higher income. The relationship between socioeconomic status and dropout rates was examined by Kruskal-Wallis testing and demonstrated no statistical significance (P = .3195), as shown in Figure 4C.
Assessment of distance traveled is also critical to understanding the financial burden associated with clinical trials and health care costs. Using Kruskal-Wallis testing, no significant findings were associated with distance traveled (P > .7875) (Figure 4B). According to National Institutes of Health guidelines, the BMI categories are as follows: underweight, less than or equal to 18.5; normal weight, 18.5 to 24.9; overweight, 25 to 29.9, and obesity, greater than or equal to 30.16 It is worth noting that increased BMI (≥30) was associated with higher dropout rates; this finding was statistically significant (P = .0304) (Figure 4D). This may be an indicator of higher disease burden in the study population that created an obstacle to trial participation.
Discussion
Clinical trials focusing on DFUs and VLUs have large patient populations that experience the effect of various SDoH. For clinical research sites involved in such trials, it is important to continually evaluate the needs of these patients. Not addressing SDoH such as race, gender, language, socioeconomic status, and travel distance to the clinical site not only may lead to issues with data integrity and quality but may also increase patients’ burden to access innovative wound care treatment options offered at the intersection of routine clinical care and clinical trials as more cutting-edge wound care products evolve.
If researchers do not account for SDoH and do not address barriers to care when conducting studies, resulting in an insufficiently diverse clinical trial population, concerns about unethical research practices and generalizability of results may arise.
The lack of statistical significance of the analyses in the present study emphasizes that the studied research site has created opportunities for diverse populations to enter clinical trials, has decreased the burden to study participation, and has continued working on equity as part of the organization’s mission in the safety net hospital environment.
Overall, based on these study findings, no statistically significant correlations were identified between examined SDoH and study protocol deviations, which allows the authors of the present study to suggest that the institution studied is on the right track to proactively manage social issues in its diverse patient population. More studies are needed to further examine site practices and develop improvement strategies to address social issues and create opportunities for diverse populations to participate in clinical trials.
To begin with, as part of the inclusive culture at the safety net hospital studied, strategies were implemented with the aim of engaging with all stakeholders (eg, sponsors, patients, ancillary services) early in the process in order to identify barriers or gaps, a Medicare coverage analysis was initiated to help determine up front what costs may be associated with participation, patient burden was addressed by providing assistance with associated costs (eg, transportation), and fully translated study documents and interpreter services were offered to non-
English-speaking research subjects (eg, follow-up reminder calls by the study coordinator to keep in touch with participants and engage them more to mitigate protocol deviations, including for non-English speakers). Based on the typical patient population, besides English, other primary spoken languages included Spanish, Haitian Creole, and Portuguese; certified interpreter services are available in person and/or over the phone at the institution studied. In addition, per IRB policies and procedures all relevant study documentation must be translated and validated for accuracy by certified translation services, such as informed consent forms, recruitment scripts, study questionnaires, pain scales, and any other patient-facing materials or tools used for data collection and assessments used in clinical research studies. While these strategies are beneficial, it is important to remain vigilant because new concerns may arise.
It is important to continuously analyze trends in protocol compliance throughout the life cycle of a project in order to develop effective strategies to better conduct trials. Trends in deviations related to missed procedures could complicate wound healing. A variety of factors may affect rate of deviations based on study protocol complexity, product indication, overall health of research participants, experience of investigators and study coordinators, etc. Continuous monitoring of study protocol adherence can lead to better outcomes and increased data quality and integrity.
Limitations
The present study has limitations. The data were attained at a single academic clinical site that is affiliated with a safety net, inner-city hospital. Thus, specific features may be attributed to the research practices and/or processes at the research site. To improve the conduct of clinical trials, and to ensure data quality and integrity, larger prospective studies should be conducted in different research settings to further examine the differences between and unique features of various practices in which clinical research is conducted, such as large academic and tertiary hospitals, private practices, and urban versus rural clinics.
Conclusion
Further studies are warranted to investigate how study protocol compliance can be enhanced with implementation of up-front training of clinical and study personnel, as well as with more efficient identification of patients who need more resources based on their SDoH. A proactive approach and adequate risk-based monitoring of key quality and risk indicators can lead to a decrease in protocol deviations and can preserve data integrity, improve compliance and quality of conducted research projects, improve patient safety, and aid in adequate resource allocation. Close supervision of studies in populations with a high disease burden and associated SDoH is important for the reduction of deviations and for quality improvement.
Author and Public Information
Authors: Nolan Patrick Joyce, MPH1; Aniket Vazirani, MD2; Connor Roddy, MS1; and Marina A. Malikova, PhD, MSci, MACI, MBA1
Affiliations: 1Department of Surgery, Boston Medical Center, Boston University, Boston, MA, USA; 2General Surgery Residency Program, Jefferson Abington Hospital, Philadelphia, PA, USA
Correspondence: Marina A. Malikova, PhD; Department of Surgery, Boston Medical Center, 85 East Concord St, Boston, MA, 02118; mmalikov@bu.edu
Disclosure: The authors have no financial or other conflicts of interest to disclose.
Ethical Approval: All clinical trials were registered through a public trials registry and received an IRB approval prior to the study initiation. Informed consent from each research subject was obtained prior to any research procedures were performed.
Manuscript Accepted: February 5, 2025
References
1. Chan B, Cadarette S, Wodchis W, Wong, J, Mittmann N, Krahn M. Cost-of-illness studies in chronic ulcers: a systematic review. J Wound Care. 2017;26(sup4):S4–S14. doi:10.12968/jowc.2017.26.Sup4.S4
2. Ongarora BG. Recent technological advances in the management of chronic wounds: a literature review. Health Sci Rep. 2022;5(3):e641. doi:10.1002/hsr2.641
3. Milic DJ, Zivic SS, Bogdanovic DC, Karanovic ND, Golubovic ZV. Risk factors related to the failure of venous leg ulcers to heal with compression treatment. J Vasc Surg. 2009;49(5):1242–1247. doi:10.1016/j.jvs.2008.11.069
4. Ghooi RB, Bhosale N, Wadhwani R, Divate P, Divate U. Assessment and classification of protocol deviations. Perspect Clin Res. 2016;7(3):132–136. doi:10.4103/2229-3485.184817
5. E6 (R3) Good Clinical Practice (GCP). Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. July 2023. FDA-2023-D-1955. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e6r3-good-clinical-practice-gcp
6. FDA Final Guidance E8(R1) General Considerations for Clinical Studies. Center for Drug Evaluation and Research. April 2022. FDA-2019-D-3049. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e8r1-general-considerations-clinical-studies
7. Getz K, Smith Z, Jain A, Krauss R. Benchmarking protocol deviations and their variation by major disease categories. Ther Innov Regul Sci. 2022;56(4):632–636. doi:10.1007/s43441-022-00401-4
8. Department of Health, Education, and Welfare; National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report. Ethical principles and guidelines for the protection of human subjects of research. J Am Coll Dent. 2014;81(3):4-13.
9. Alsan M, Wanamaker M, Hardeman RR. The Tuskegee Study of Untreated Syphilis: a case study in peripheral trauma with implications for health professionals. J Gen Intern Med. 2020;35(1):322-325. doi:10.1007/s11606-019-05309-8
10. Skloot R. The Immortal Life of Henrietta Lacks. Audiobook. Random House Audio; 2010.
11. Food and Drug Omnibus Reform Act of 2022 (FDORA). https://www.govinfo.gov/content/pkg/BILLS-117hr2617enr/pdf/BILLS-117hr2617enr.pdf
12. World Health Organization. Social Determinants of Health. Retrieved April 12, 2025. https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1
13. United States Census Bureau data. https://data.census.gov/all?q=Income+by+zip+code
14. Spearman C. The proof and measurement of association between two things. Am J Psychol. 1904;15(1):72–101. doi:10.2307/1412159
15. Lehman A, O’Rourke N, Hatcher L, Stepanski EJ. JMP for Basic Univariate and Multivariate Statistics: A Step-by-Step Guide. 1st ed. SAS Press; 2005.
16. NIH BMI: Assessing Your Weight and Health Risk. https://www.nhlbi.nih.gov/health/educational/lose_wt/risk.htm
















