The Effect of Hyperbaric Oxygen Therapy on Split-Thickness Skin Graft Uptake in Posttraumatic Wounds and Donor Site Healing: A Randomized Controlled Trial
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. Trauma is among the leading causes of skin loss or degloving. Flaps and skin grafts are common surgical procedures to repair or replace the lost skin over open wounds, and split-thickness skin grafting (STSG) is the most common approach. Objective. To assess the effect of hyperbaric oxygen therapy (HBOT) on STSG uptake and donor site healing. Materials and Methods. This trial was conducted on patients with traumatic wounds who underwent STSG as per inclusion criteria. The patients were randomized into 2 groups. One group received standard care after skin grafting, and the other received HBOT in addition to standard care. Uptake of STSG was assessed on postoperative day (POD) 4 and POD 7, and donor site healing was assessed on POD 11 and POD 15. Results. A total of 64 patients aged 18 years to 60 years were included in the study. Mean (standard deviation [SD]) percentage graft uptake on POD 4 was 92.44% (5.98%) in the HBOT group and 88.12% (8.92%) in the control group (P = .036), and on POD 7 was 91.69% (8.71%) in the HBOT group and 83.12% (14.94%) in control group (P = .026). Donor site recovery was also significantly faster in the HBOT group, with a mean (SD) of 15.16 (0.88) days in the HBOT group and 17.97 (2.49) days in the control group (P < .001). In the control group, floating grafts were found in 2 patients, flap necrosis occurred in 4 patients, and 1 patient died due to sepsis, whereas in the HBOT group, significant graft contracture and wound recipient site infection occurred in 1 patient each. Conclusion. HBOT significantly improved the percentage graft uptake in posttraumatic wounds and resulted in better donor site healing compared with standard care alone.
Abbreviations: ATA, atmospheres absolute; HBOT, hyperbaric oxygen therapy; Nrf2, nuclear factor erythroid 2–related factor 2; POD, postoperative day; SD, standard deviation; STSG, split-thickness skin graft; TEWL, transepidermal water loss
Background
Extremity trauma is among the most frequent reasons for loss of productivity.1 Many patients with extremity trauma need flap coverage or skin grafting. In trauma patients, coverage of open wounds with STSG is a commonly used surgical procedure.2 Similar to healing of other soft tissues, the uptake of STSG is influenced by many factors. One of the most important factors is tissue oxygen level.3 In a compromised graft or wound bed, improved oxygen perfusion at a cellular level and avoidance of infection are essential for graft uptake and survival. HBOT improves the tissue oxygen levels, leading to adequate amounts of mature collagen formation.4
HBOT has both direct and indirect effects.5 Primary effects include increase in pressure and hyperoxia by up to 20 times. During HBOT, hyperoxygenation occurs as a result of Henry’s law, which states that the amount of gas dissolved in a liquid is directly proportional to the partial pressure of the gas.6 Dissolved oxygen in plasma is increased due to elevated arterial partial pressure of oxygen. At a pressure of 3 ATA, 6 mL of oxygen is dissolved for every 100 mL of plasma, providing the same amount of oxygen delivery as oxygen bound to hemoglobin.6 Additional benefits of HBOT include antibacterial activity, blunting of ischemic-reperfusion injury, reducing vasogenic edema after trauma, improving mobilization of endothelial progenitor cells, and wound healing due to regulated oxidative stress. Wound healing thus depends on systemic factors as well as effects at the site of injury.7
Donor site healing consists of reepithelialization of the site from which the graft is taken, which occurs by means of cells migrating from the remnants of hair follicles, sebaceous glands, and sweat glands in reticular dermis. This occurs in approximately 15 days, but may take as long as 21 days depending on age and nutritional status of the patient.8 Clinically, the donor site appears dry, is painless, and is covered by epithelium. To the authors’ knowledge, no published data are available evaluating the effect of HBOT on graft donor site healing.
This randomized controlled trial assessed the effect of HBOT on the uptake of STSG in posttraumatic wounds and on donor site healing.
Materials and Methods
Type of study and sample size
This clinical trial was conducted in the Department of Trauma Surgery & Critical Care of the All India Institute of Medical Sciences Rishikesh in India. Ethical approval was received from that institution’s Institutional Ethical Committee, and the trial was registered in Clinical Trial Registry-India (www.ctri.nic.in; CTRI REF/2020/10/037195). Written informed consent was obtained from all the participants after thoroughly explaining the research project in their language.
This was a single-masked (ie, operating surgeon masked to the patient treatment category), parallel randomized controlled trial with an allocation ratio of 1:1, designed as a study of therapeutic intervention (HBOT in addition to standard care vs standard care only) in patients requiring STSG of wounds due to trauma. The trial was conducted from December 2019 through August 2021. Sample size was established using a power calculation based on variance established by previous studies regarding skin graft uptake; with 95% CI and power at 80%, the sample size was estimated to be 56, with SD of outcome variable at 4 using the formula
N = 2 σ2 (z1−β+z1−α/2)2
(µ0−µ1)2
Fixed block randomization with a size of 4 blocks without any specific bias was used. The randomization and participant assignment were done by a clinician other than the operating surgeon, whereas enrollment was done by all members of the treating team.
After randomization, the participants were categorized into 2 groups (Figure 1). The primary objective was to compare STSG uptake in patients receiving standard postoperative care with uptake in patients receiving HBOT in the postoperative period. The secondary objective was to compare donor site healing between controls and those receiving HBOT. The control group received standard postoperative care, which includes nonadherent dressings, limb immobilization and elevation, as well as antibiotics and analgesics. The HBOT group received therapy at atmospheric pressure of 1.5 to 2 ATA for 90 minutes in daily sessions from POD 2. All patients received antibiotics and analgesics as per departmental protocol irrespective of group allocation. Serial wound examination was done by a clinician other than the operating surgeon. Recipient site examination was done on POD 4 and POD 7 for assessment of graft uptake percentage.
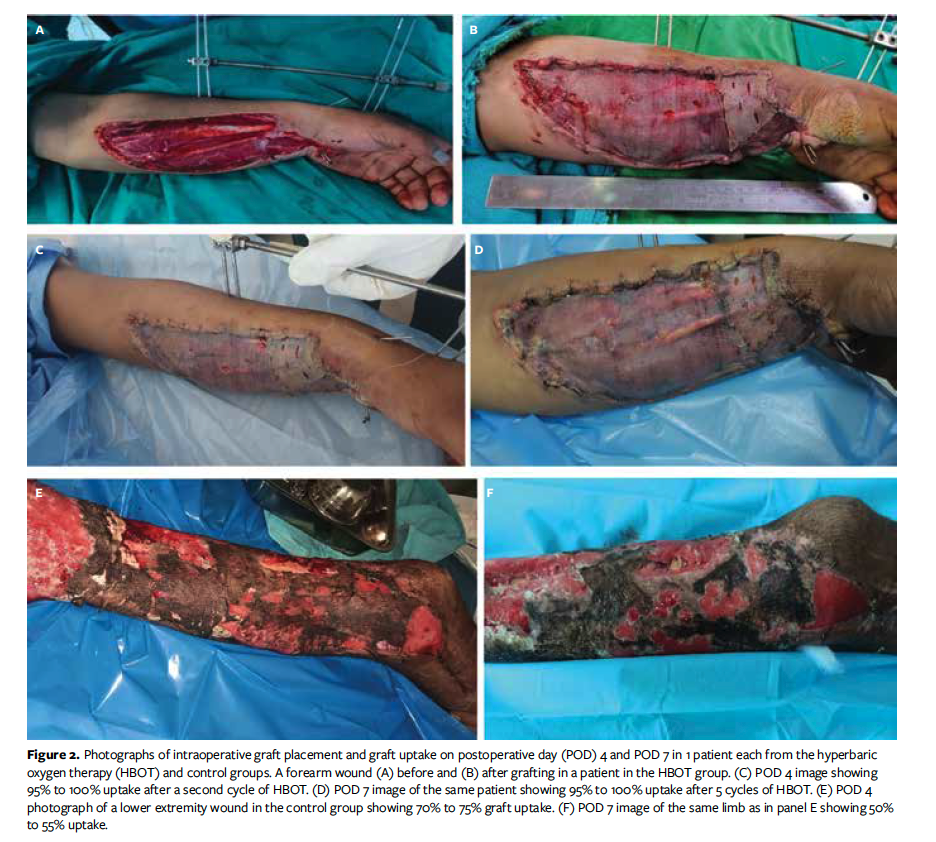
Graft uptake percentage was calculated using an acetate tracing method in which a 2-layered transparent acetate sheet with a 1-cm2 grid pattern is placed over the grafted area and the graft margins are traced using a permanent pen or marker and the squares are calculated. Acetate tracing was done by the clinician other than the operating surgeon and was done by the same person each time. Donor site examination was done on POD 11 and POD 15 (followed by daily examinations in patients with incomplete healing) for clinical assessment of donor site healing. The operating surgeon was masked regarding to which treatment category the patient was allotted.
Data analysis was done using SPSS version 25.0 (IBM Corporation), after data were coded and recorded in an Excel spreadsheet (Microsoft). Descriptive statistics were presented as mean (SD) and median (IQR) for continuous variables, and as frequencies and percentages for categorical variables. The Mann-Whitney U (Wilcoxon) test was used for nonparametric comparison, Spearman rank correlation was used for nonparametric correlation, and the Fisher exact test was used for association between 2 groups. A P value less than .5 was deemed statistically significant.
Definitions
STSGs are classified according to thickness as thin (0.15 mm–0.3 mm), intermediate (0.3 mm–0.45 mm), and thick (0.45 mm–0.6 mm).9 Meshing can be done to expand the harvested skin grafts to ratios ranging from 1:1 to 6:1 or higher depending on the size of recipient area and available donor sites. The authors of the present study arbitrarily defined “primary” STSG as that done on fresh wounds within 24 hours of injury. “Secondary” STSG was defined as grafting after serial debridement and dressings and/or after the appearance of healthy granulation tissue, which usually takes longer than 24 hours.
Inclusion and exclusion criteria
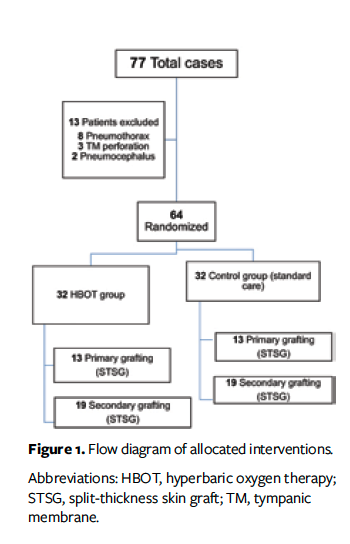
Inclusion criteria consisted of patients aged 18 years to 60 years undergoing STSG for posttraumatic wounds. The following patients were excluded: patients undergoing STSG for wounds other than traumatic wounds; those with polytrauma (ie, ≥2 severe injuries in at least 2 areas of the body); and those with contraindications to HBOT, such as pneumothorax, pneumocephalus, tympanic membrane perforation, claustrophobia, and ejection fraction less than 30%.
Results
The study population was aged 18 years to 60 years. A total of 77 patients underwent STSG during the study period; 13 of these patients were excluded due to contraindications to HBOT (8 patients had pneumothorax, 3 had tympanic membrane perforation, and 2 had pneumocephalus). Of the remaining 64 patients, 32 were allocated to the HBOT group and 32 to the control group. All patients completed the required follow-up (there were no losses). Of the 64 patients, 54 were males (29 in the control group and 25 in the HBOT group) and 10 were females (3 in the control group and 7 in the HBOT group). Age, length of hospital stay, and comorbidities were comparable in both groups (Table). A total of 26 patients (40.6%) underwent primary skin grafting, and 38 (59.4%) underwent secondary skin grafting. The mean (SD) wound size was 110.10 cm² (91.05 cm²) in the HBOT group and 160.58 cm² (364.93 cm²) in the control group, with no statistical difference as per the Mann-Whitney U (Wilcoxon) test (P > .99). Mean percentage graft uptake was 92.44% (5.98%) in the HBOT group and 88.12% (8.92%) in the control group on POD 4 (P = .036) and was 91.69% (8.71%) in the HBOT group and 83.12% (14.94%) in the control group on POD 7 (P = .026). The mean time to donor site healing was 15.16 (0.88) days in the HBOT group and 17.97 (2.49) days in the control group (P < .001).
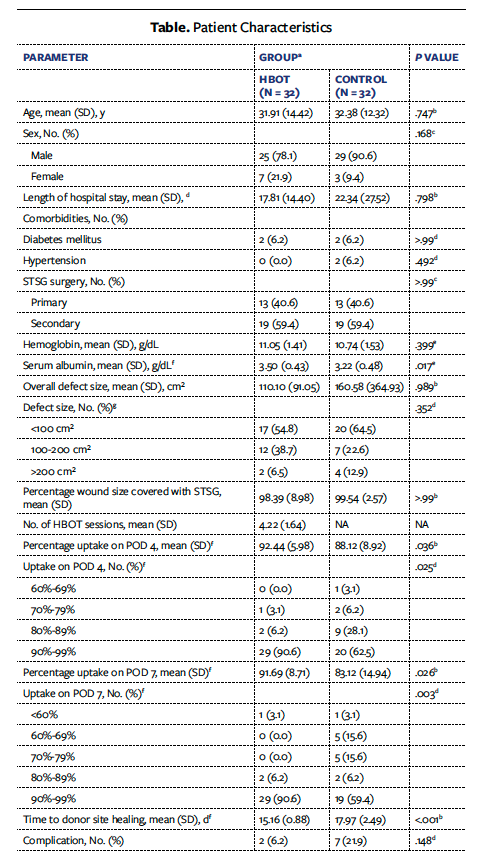
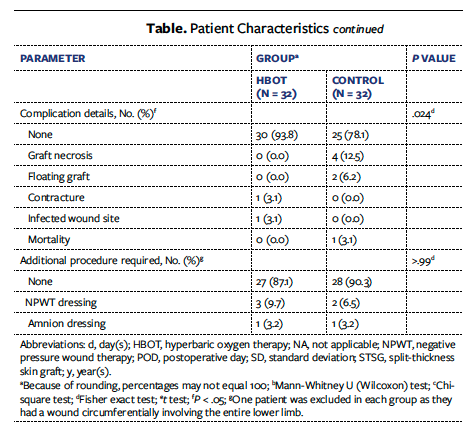
It was also observed that 40 patients with hemoglobin level of 9 g/dL to 11 g/dL had greater than 90% skin graft uptake. Eight of these patients had 80% to 89% skin graft uptake, and 2 patients had 70% to 79% skin graft uptake. For every 1-unit increase in hemoglobin (g/dL) above 7 g/dL, the uptake increased by 2.21%. It was also found that a serum albumin level above 3 g/dL resulted in skin graft uptake of 92.45%, while a serum albumin level below 3 g/dL resulted in skin graft uptake of 81.77%. For every 1-unit increase in serum albumin (g/dL) above 3 g/dL, uptake increased by 9.55%. In the HBOT group, 1 patient had contracture and 1 had infection. More than 4 (mean [SD], 4.22 [1.64]) sessions of HBOT were needed to significantly increase the STSG uptake in patients with traumatic wound defects. In the control group, floating grafts were found in 2 patients, flap necrosis occurred in 4 patients, and 1 patient died due to sepsis, whereas in the HBOT group, significant graft contracture and wound recipient site infection occurred in 1 patient each. Additional procedures in the form of negative pressure wound therapy were required in 3 patients in the HBOT group and 2 patients in the control group, and amnion dressing was needed in 1 patient in each group.
Discussion
HBOT has been shown to have physiological effects that accelerate wound healing. This is attributed to better diffusion of oxygen at high atmospheric pressures. A substantial amount of work has been done demonstrating the significance of the role of HBOT in tissue healing.10-14 The various reported mechanisms by which HBOT improves healing include direct activation of Nrf2 protein expression; an increase in heme oxygenase-1, epidermal growth factor, vascular endothelial growth factor, platelet-derived growth factor, fibroblast growth factor 2, endothelial nitric oxide, and interleukin 88,10-15; increased angiogenesis16-18; reduction of endothelial intercellular adhesion molecule 1 and vascular cell adhesion molecule 119; blunting of ischemic-reperfusion injury10; and increasing hypoxia-inducible factor-1α stability and activity, thereby inducing fibroblast proliferation.20,21
Some studies have shown a positive effect of HBOT on healing and survival of flap and grafts.16,22 Isolated studies on the role of HBOT on STSG survival exist, and to the knowledge of the authors of the present article, almost none of them have addressed the effect of HBOT on donor site healing. Baynosa et al10 reviewed the role of HBOT on graft and flap survival and concluded that there is a paucity of evidence in the literature demonstrating the role of HBOT on STSG uptake.
The present study not only demonstrates the role of HBOT on STSG uptake but also discusses the simultaneous role of HBOT on donor site healing—a component that is as essential as graft healing. Mean (SD) percentage graft uptake on POD 4 and POD 7 was 92.4% (5.98%) and 91.69% (8.71%), respectively, in the HBOT group vs 88.12% (8.92%) and 83.12% (14.94%), respectively, in the control group (Figures 2 and 3).
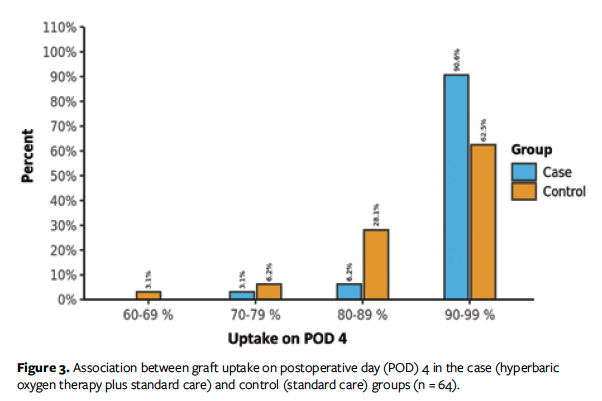
In 1967, Perrins and Cantab23 reported permanent mean (SD) graft survival of 84% (2%) for the HBOT group and 62% (7%) for the control group. In their prospective, randomized controlled trial to examine the effect of HBOT on STSG, they found that 64% of people who received HBOT had complete skin graft uptake (ie, >95% of the surviving graft area), while only 17% of patients in the control group experienced the same result. In addition, all patients who received HBOT had greater than 60% graft uptake, while only 64% of the control group had greater than 60% graft uptake. Thirty-one of the 32 patients in the HBOT group had greater than 80% graft uptake, whereas 12 of 32 patients in the control group had 50% to 80% graft uptake.23
Roje et al,24 in a retrospective study, reported substantially greater loss of skin grafts in the control group than in the HBOT group (52% and 23%, respectively). Zhou et al22 conducted a large review of RCTs, including 23 clinical trials with a total of 957 patients who received HBOT and 583 the control group. They reported an overall graft survival rate of 62.5% to 100% in the HBOT group compared with 35.0% to 86.5% in controls, especially when STSG was conducted within 72 hours of surgery.
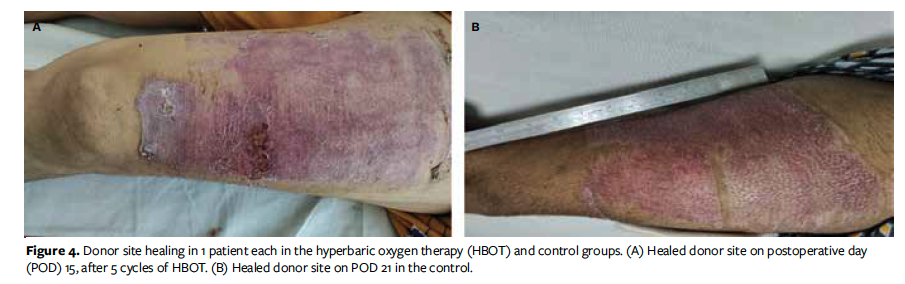
In the present study, donor site healing completed within a mean (SD) of 15.16 (0.88) days in the HBOT group and 17.97 (2.49) days in the control group (Figure 4). The authors of the present study could not find any literature on the effect of HBOT on donor site healing. Histopathologic examination is the ideal way to assess for complete epithelialization, or closure, of donor sites; however, it is an invasive procedure and is cumbersome for patients. Clinical examination is still the most basic and common method of assessing donor site closure. TEWL is also used for assessment of skin epithelialization. However, TEWL is also affected by multiple factors, such as hydration, temperature, and humidity. In addition, TEWL was not available at AIIMS Rishikesh. Brölmann et al25 reported shorter time to complete reepithelialization using hydrocolloid dressings compared with other dressing materials (median 16 vs 23 days, respectively). Kale et al26 conducted a retrospective observational study on the effect of hemoglobin on uptake of skin grafting and found no statistically significant difference between patients with anemia and those without anemia. In the present study, 40 patients had a hemoglobin level of 9 g/dL to 11 g/dL; of these, 30 patients had greater than 90% uptake of skin graft, 8 had 80% to 89% uptake, and 2 had 70% to 79% uptake.
Limitations
The limitations of the present study include small sample size and the use of acetate tracing to measure percentage graft uptake. Acetate tracing has some limitations, including difficulty in accurately measuring the size of small or irregular wounds; it is also time-consuming.
Conclusion
The present study demonstrated a significant increase in percentage STSG uptake and better outcome in patients who received HBOT in addition to standard care compared with standard care alone. Early donor site healing was also observed in the HBOT group as compared with the control group. However, more than 4 (mean [SD], 4.22 [1.64]) sessions of HBOT were required to achieve a significant increase in STSG uptake in patients with traumatic wounds. Additionally, a hemoglobin level greater than 9 g/dL and a serum albumin level greater than 3g/dL was associated with increased STSG uptake.
Author and Public Information
Authors: Madhur Uniyal, MCh1; Irshad Ahmad, MCh1; Ajay Kumar Dhiman, MCh2; Ajay Kumar, MS3; Bhaskar Sarkar, MS1; Nilesh Jagne, MCh4; Vishal Mago, MCh5; and Md Quamar Azam, MS1
Affiliations: 1Department of Trauma Surgery & Critical Care, All India Institute of Medical Sciences, Rishikesh, India; 2Department of General Surgery, All India Institute of Medical Sciences, Bilaspur, India; 3Department of General Surgery, Rajendra Institute of Medical Sciences, Ranchi, India; 4Department of Trauma and Emergency, All India Institute of Medical Sciences, Nagpur, India; 5Department of Burns and Plastic Surgery, All India Institute of Medical Sciences, Rishikesh, India.
Author Contributions: M.U.: conceptualization, methodology; I.A.: manuscript editing and revision, resources; A.K.D.: data curation; A.K. and B.S.: formal analysis; N.J.: Patient selection, investigation, resources, writing original manuscript; and corresponding and primary author of this manuscript; V.M.: project administration; M.Q.A.: supervision. M.U. and I.A. contributed equally to this manuscript and share joint first authorship.
Ethical Approval: Ethical approval was received from the ethical committee of AIIMS Rishikesh.
Disclosure: The authors have no financial or other conflicts of interest to disclose. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
Correspondence: Nilesh Jagne, MCh; Department of Trauma and Emergency, All India Institute of Medical Sciences (AIIMS), Maharashtra 441108, Nagpur, India; nileshjagne@gmail.com
Manuscript Accepted: February 5, 2025
References
1. Devendra A, Nishith PG, Raja SDC, Dheenadhayalan J, Rajasekaran S. Current updates in management of extremity injuries in polytrauma. J Clin Orthop Trauma. 2021;12(1):113-122. doi:10.1016/j.jcot.2020.09.031
2. Sakai G, Suzuki T, Hishikawa T, Shirai Y, Kurozumi T, Shindo M. Primary reattachment of avulsed skin flaps with negative pressure wound therapy in degloving injuries of the lower extremity. Injury. 2017;48(1):137-141. doi:10.1016/j.injury.2016.10.026
3. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219-229. doi:10.1177/0022034509359125
4. Atiyeh BS, Gunn SW, Hayek SN. State of the art in burn treatment. World J Surg. 2005;29(2):131-148. doi: 10.1007/s00268-004-1082-2
5. Braswell C, Crowe DT. Hyperbaric oxygen therapy. Compend Contin Educ Vet. 2012;34(3):E1-6.
6. Bhutani S, Vishwanath G. Hyperbaric oxygen and wound healing. Indian J Plast Surg. 2012;45(02):316-324. doi:10.4103/0970-0358.101309
7. Heyboer III M, Sharma D, Santiago W, McCulloch N. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6(6):210-224. doi:10.1089/wound.2016.0718
8. Seyhan T. Split-thickness skin grafts. In: Spear M, ed. Skin Grafts-Indications, Applications and Current Research. Intech Open; 2011. doi:10.5772/23658
9. Braza ME, Fahrenkopf MP. Split-thickness skin grafts. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK551561
10. Baynosa RC, Naig AL, Murphy PS, et al. The effect of hyperbaric oxygen on nitric oxide synthase activity and expression in ischemia-reperfusion injury. J Surg Res. 2013;183(1):355–361. doi:10.1016/j.jss.2013.01.004
11. Meng XE, Zhang Y, Li N, et al. Effects of hyperbaric oxygen on the Nrf2 signaling pathway in secondary injury following traumatic brain injury. Genet Mol Res. 2016;15(1). doi:10.4238/gmr.15016933
12. Dhamodharan U, Karan A, Sireesh D, et al. Tissue-specific role of Nrf2 in the treatment of diabetic foot ulcers during hyperbaric oxygen therapy. Free Radic Biol Med. 2019;138:53–62. doi:10.1016/j.freeradbiomed.2019.04.031
13. Goldstein LJ, Gallagher KA, Bauer SM, et al. Endothelial progenitor cell release into circulation is triggered by hyperoxia-induced increases in bone marrow nitric oxide. Stem Cells. 2006;24(10):2309–2318. doi:10.1634/stemcells.2006-0010
14. Boykin JV Jr, Baylis C. Hyperbaric oxygen therapy mediates increased nitric oxide production associated with wound healing: a preliminary study. Adv Skin Wound Care. 2007;20(7):382–388. doi:10.1097/01.ASW.0000280198.81130.d5
15. Gajendrareddy PK, Sen CK, Horan MP, Marucha PT. Hyperbaric oxygen therapy ameliorates stress-impaired dermal wound healing. Brain Behav Immun. 2005;19(3):217–222. doi:10.1016/j.bbi.2004.09.003
16. Gill AL, Bell CNA. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM. 2004;97(7):385-395. doi:10.1093/qjmed/hch074
17. von Kessel F. Influence of hyperbaric oxygen and irradiation on vascularity in skin flaps: a controlled study. Plast Reconstr Surg. 1990;85(3):489.
18. Godman CA, Chheda KP, Hightower LE, Perdrizet G, Shin DG, Giardina C. Hyperbaric oxygen induces a cytoprotective and angiogenic response in human microvascular endothelial cells. Cell Stress Chaperones. 2010;15(4):431–442. doi:10.1007/s12192-009-0159-0
19. Kendall AC, Whatmore JL, Winyard PG, Smerdon GR, Eggleton P. Hyperbaric oxygen treatment reduces neutrophil-endothelial adhesion in chronic wound conditions through S-nitrosation. Wound Repair Regen. 2013;21(6):860–868. doi:10.1111/wrr.12108
20. Sunkari VG, Lind F, Botusan IR, et al. Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice. Wound Repair Regen. 2015;23(1):98–103. doi:10.1111/wrr.12253
21. Zhu Y, Wang Y, Jia Y, Xu J, Chai Y. Roxadustat promotes angiogenesis through HIF-1α/VEGF/VEGFR2 signaling and accelerates cutaneous wound healing in diabetic rats. Wound Repair Regen. 2019;27(4):324–334. doi:10.1111/wrr.12708
22. Zhou YY, Liu W, Yang YJ, Lu GD. Use of hyperbaric oxygen on flaps and grafts in China: analysis of studies in the past 20 years. Undersea Hyperb Med. 2014;41(3):209–216.
23. Perrins DJ, Cantab MB. Influence of hyperbaric oxygen on the survival of split skin grafts. Lancet. 1967;289(7495):868-871. doi:10.1016/s0140-6736(67)91428-6
24. Roje Z, Roje Ž, Eterović D, et al. Influence of adjuvant hyperbaric oxygen therapy on short-term complications during surgical reconstruction of upper and lower extremity war injuries: retrospective cohort study. Croat Med J. 2008;49(2):224-232. doi:10.3325/cmj.2008.2.224
25. Brölmann FE, Eskes AM, Goslings JC, et al. Randomized clinical trial of donor-site wound dressings after split-skin grafting. Br J Surg. 2013;100(5):619-627. doi:10.1002/bjs.9045
26. Kale AR, Sonawane CS, Wagh AA, Mangukiya HJ, Waghmare VU. A retrospective study of effect of anaemia on split thickness skin graft uptake in orthopaedic trauma cases. International Journal of Orthopaedics Sciences. 2017;3(4):562-564. doi:10.22271/ortho.2017.v3.i4h.78


















