Bromelain-Based Debridement Versus Collagenase Ointment Debridement of Venous Leg Ulcers: Post Hoc Analysis of the ChronEx Trial
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. A randomized controlled trial reported that bromelain-based enzymatic debridement (BBD) more effectively debrided and granulated venous leg ulcers (VLUs) compared with placebo (gel vehicle, hydrogel) and nonsurgical standard of care (including collagenase ointment [CO]). Objective. To assess the efficacy of BBD vs CO-based enzymatic debridement in VLUs during the ChronEx trial. Materials and Methods. The Wilcoxon exact test was used to compare the proportion of wounds in each group that achieved complete debridement and granulation at 2 weeks. Kaplan-Meier analysis was used to compare median times to complete debridement and granulation between groups. Results. Forty-six patients with 46 wounds were treated with BBD, and 8 were treated with CO. Twenty-nine wounds treated with BBD (63%; 95% CI, 48–77) were completely debrided within 2 weeks compared with none treated with CO (P = .001). Twenty-three wounds treated with BBD (50%; 95% CI, 35–65) achieved complete granulation by 2 weeks compared with none with CO (P = .015). The estimated median time to complete debridement and complete granulation, respectively, in the BBD group vs the CO group, respectively, were 9 days vs not achieved (P = .023), and 11 days vs not achieved (P = .014). The groups had comparable safety and pain profiles. Conclusion. BBD appears to be more effective and faster than CO in achieving complete debridement and granulation of VLUs as part of wound bed preparation.
Abbreviations: AE, adverse event, BBD; bromelain-based enzymatic debridement; CO, collagenase ointment; DFU, diabetic foot ulcer; GV, gel vehicle; ITT, intention-to-treat; mITT, modified ITT; NERDS, nonhealing wound, exudative wound, red and bleeding wound, debris in the wound, smell from the wound; NSSOC, nonsurgical standard of care; RCT, randomized controlled trial; RMST, restricted mean survival time; SD, standard deviation; SMG, saline-moistened gauze; STONES, size is bigger, temperature increased, os (probes to or exposed bone), new areas of breakdown, exudate, erythema or edema, smell; VLU, venous leg ulcer.
For more than 5 decades, CO derived from the Clostridium histolyticum bacteria (SANTYL, Smith+Nephew) has been used as a topical, enzymatic debridement agent, mainly in conjunction with sharp debridement.1 CO specifically degrades the triple helix of native type 1 collagen, which is abundant in wound eschar and slough. This targeted action facilitates the removal of devitalized tissue from the wound bed.2-7 Furthermore, research suggests that collagen cleavage by CO releases peptide fragments that may stimulate proliferation and migration of keratinocytes and fibroblasts.5-10 A systematic review and meta-analysis of 22 RCTs evaluating the effect of CO on debridement concluded that CO appears to be beneficial in the management of pressure injuries and DFUs; however, high-quality RCTs are lacking, and data are insufficient to support the use of CO in chronic VLUs.11
BBD (EscharEx, MediWound Ltd) is a new biological drug currently under development for debridement of VLUs and DFUs. The active material in BBD is a complex mixture of proteolytic enzymes enriched in bromelain extracted from the pineapple stem. The active material in BBD is identical to anacaulase-bcdb, the active pharmaceutical ingredient in NexoBrid (MediWound Ltd), an enzymatic agent for eschar removal, the latter of which is approved in more than 40 countries, including the United States and the European Union, for eschar removal (debridement) of deep partial- and/or full-thickness thermal burns.1,12 However, the final formulation of the 2 products is substantially different. Anacaulase-bcdb (a powder containing BBD mixed with a gel to form 10% BBD concentration) has been shown to be faster than and similarly effective as standard of care for eschar debridement.1,12 Due to its strong affinity to denatured collagen, anacaulase-bcdb selectively debrides injured dermis only, leaving healthy dermis undisturbed.13 Healthy dermis can thus heal spontaneously by epithelialization with reduced need for autografting.14,15 The enzymatic mixture composition of BBD is formulated to enable effective debridement of denatured proteins and nonvital tissues that are commonly found in VLUs, DFUs, and other hard-to-heal wounds, including denatured collagen, elastin, and fibrin.13
BBD efficacy was initially demonstrated in porcine ischemic wound models,16-18 followed by 3 phase II clinical trials that enrolled patients with VLUs, DFUs, and posttraumatic hard-to-heal wounds.12,19,20
In an RCT in which a powder containing BBD mixed with a gel to form 5% or 2.5% BBD concentration was compared with the gel vehicle alone (GV, powder without the active ingredient mixed with a gel) in 73 patients with chronic wounds, the proportion of wounds with complete debridement was significantly higher in the BBD group compared with the placebo gel vehicle group (55% and 29%, respectively; P = .047).19 BBD was next reformulated to optimize utility and increase treatment application time from 4 hours to 24 hours; a powder containing BBD and excipients was mixed with water for injection to form 5% BBD concentration. In a phase II study of the effect of BBD on 12 patients with chronic VLU or DFU, complete debridement occurred in 7 patients with up to 8 once-daily treatments; punch biopsy and fluorescence imaging confirmed reduced biofilm and bacterial load.20 The original article of the ChronEx RCT was published in September 2024 and reported the safety and efficacy of BBD compared with the placebo hydrogel, hereafter referred to as the GV, and NSSOC debridement agents in 119 patients with chronic VLU.12 With up to 8 daily treatments (over a period of up to 2 weeks), complete debridement was achieved in 63% of wounds in the BBD group (29 of 46), compared with 30% (13 of 43) in the GV group (P = .004) and 13% (4 of 30) in the NSSOC group (P < .001). Moreover, the median time to complete debridement was 9 days with BBD treatment, 63 days with the GV, and 59 days with NSSOC. The incidence of complete cover of the wound bed with healthy granulation tissue during the daily treatment period was 50% (23 of 46) for BBD, compared with 26% (11 of 43) for placebo (P = .01) and 10% (3 of 30) for NSSOC (P < .001). BBD shared a similar safety profile with the other groups, in terms of AEs and pain levels. In the NSSOC group, 27% of patients (8 of 30) received CO.12
Because CO is the only commercially available enzymatic debridement agent for chronic wounds, the authors of the present study wanted to further explore the comparative effectiveness of BBD and CO in their RCT population. Therefore, this post hoc analysis evaluates the safety and effectiveness of BBD compared with CO in the debridement of chronic VLUs.
Materials and Methods
The detailed inclusion/exclusion criteria and study methodology of the ChronEx RCT has been published previously.12 The trial was registered on ClinicalTrials.gov (no. NCT03588130) and on EudraCT (no. 2020-00486-38). Twenty wound centers in the US, Switzerland, and Israel participated in this study. The study adhered to the 1975 Declaration of Helsinki, and the institutional review boards and ethics committees of all participating institutions approved the study protocol. Patients aged 18 years to 90 years who presented to the study centers with venous insufficiency (diagnosed by medical history, physical examination, and an ultrasound) and a chronic VLU with a duration of 4 weeks to 2 years, a wound area of 2 cm2 to 100 cm2, and a wound bed having greater than 50% nonviable tissue; who provided their written informed consent; and whose wounds did not decrease in area by at least 20% during the 6- to 9-day run-in period were enrolled. Patients with arterial insufficiency (ankle-brachial index ≤0.70, toe brachial index ≤0.50, skin perfusion pressure ≤40 mm Hg, or transcutaneous oximetry ≤40 mm Hg) were excluded, as were those undergoing the following wound treatment modalities: surgical, mechanical, or biological debridement; negative pressure wound therapy; or hyperbaric oxygen therapy.
Subjects randomized to the BBD group (EscharEx EX-02 formulation, MediWound Ltd) were treated daily with topical BBD. Additionally, zinc oxide ointment (Calmoseptine, Calmoseptine Inc) was applied to protect the periwound skin and, together with an occlusive dressing (3M Tegaderm, 3M), adhered the BBD to the treatment area. Daily applications continued until complete debridement occurred (defined as achieving a viable wound bed after removal of all nonviable tissue, suitable for initiation of the wound healing stage) or the patient received up to 8 daily treatments within 2 weeks. After this initial 2-week daily treatment period, BBD application was not allowed. Patients were followed up twice weekly for 2 weeks, during which they received standardized nonactive dressings (eg, foam, hydrogel, or hydrocolloid) and grafting (including autograft, allograft, and xenograft), per investigator discretion.12 They were then followed up weekly for an additional 10 weeks. At this time, wounds in the BBD group that did not achieve complete debridement during the 2-week treatment period were allowed to undergo surgical debridement after the daily treatment period; however, they were regarded as treatment failures.
Subjects randomized to the NSSOC group underwent autolytic and/or enzymatic debridement (with CO). The CO group was treated with topical CO once daily for up to 2 weeks, until complete debridement occurred, followed by once-daily, twice-weekly, or once-weekly applications per label indications or per the investigator’s discretion, for the duration of the study period. Surgical debridement was prohibited in this group for the entire duration of the study.
Wound assessment was performed using a validated 3-dimensional wound measurement imaging device with a planimetry system (eKare, eKare Inc). Wound culture was performed at both the screening visit and the end-of-treatment visit, or as required by clinical judgment. Assessment of superficial and deep wound infection was performed using the mnemonic NERDS (for evaluating clinical signs of critical bacterial colonization) and STONES (for evaluating clinical signs of deep wound infection) methods.21 Wound closure was defined as complete epithelialization of the wound surface without drainage or need for dressing; 2 weeks after wound closure was first observed, the subject had a healing confirmation visit.
All VLUs were additionally managed with a 2-layer compression system (Coban, 3M) that was reapplied after every dressing change.
The sample size of this post hoc analysis was all randomized subjects (ITT population) in the BBD group (n = 46) and the CO subset of the NSSOC group (n = 8). In the BBD group, a mITT analysis was also performed, because 3 subjects in the BBD group underwent surgical debridement following the 2-week daily treatment period. They were censored for time points starting with the date of their surgical debridement.
The post hoc analysis focused on outcomes related to complete wound bed preparation, which was determined by achievement of the 2 essential wound bed states, that is, having a completely debrided and a completely granulated wound with healthy tissue.22 Therefore, the following efficacy end points were evaluated: proportion of wounds achieving complete debridement at 2 weeks and at the end of the study (14 weeks), proportion of wounds achieving complete granulation at 2 weeks and at 14 weeks, proportion of wounds achieving complete wound bed preparation at 2 weeks and at 14 weeks, proportion of wounds achieving complete closure at 14 weeks, as well as time to complete debridement, time to complete granulation (which served as the surrogate for complete wound bed preparation because granulation was the limiting rate factor), and time to complete closure in days. Safety end points evaluated were the pain levels reported during the previous day’s application (assessed using a score of 0 to 10, with 0 being “no pain” and 10 being “worst possible pain”) during the initial 2-week daily treatment period and the incidence and severity of AEs.
Descriptive statistics summarized continuous variables as mean (SD) because there was normal distribution. Statistical tests were performed against a 2-sided alternative hypothesis, using a significance level of .05. The Fisher exact test was used to compare incidence rates between groups, and 95% CIs were calculated using the Clopper-Pearson method. Log-rank and RMST tests were used to compare rates of time between the study groups. To analyze group differences for time to complete closure, the area under the curve was analyzed. The Kaplan-Meier survival function curve plotted the time to complete debridement, time to complete granulation, and time to complete closure. All statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc).
Results
Table 1 summarizes the patient and wound characteristics. Wound characteristics were comparable between the 2 groups, with no statistically significant differences.
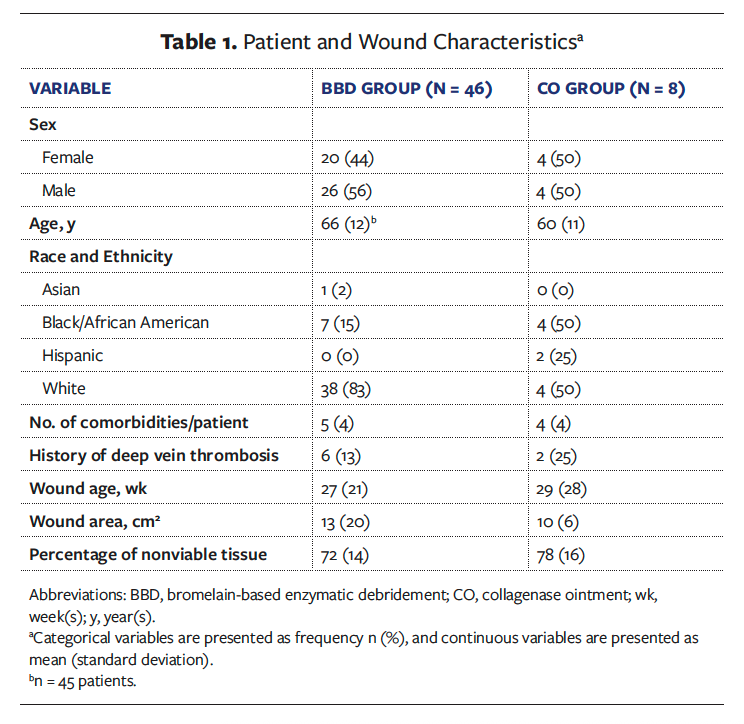
Table 2 summarizes key efficacy outcomes comparing BBD with CO for the ITT population. There were 29 wounds with complete debridement at 2 weeks in the BBD group (63%; 95% CI, 48–77), compared with none in the CO group (P = .001). There were 37 wounds in the BBD group with complete debridement at 14 weeks (80%; 95% CI, 66–91), compared with 4 (50%; 95% CI, 16–84) in the CO group. This difference was not statistically significant.
Complete granulation and wound bed preparation had the same incidence in this study because achieving wound bed preparation was dependent on complete granulation, the slower, time-limiting variable. There were 23 wounds with complete granulation/wound bed prepared at 2 weeks in the BBD group (50%; 95% CI, 35–65), compared with none in the CO group (P = .015). There were 36 wounds in the BBD group with complete granulation/wound bed prepared throughout the study (up to 14 weeks) (78%; 95% CI, 64–81), compared with 3 (38%; 95% CI, 9–76) in the CO group (P = .030).
A slightly higher percentage of wounds closed within 14 weeks in the BBD group compared with the CO group (33% [n = 15] and 25% [n = 2], respectively). This difference was not statistically significant.
A mean (SD) of 5 (2.5) BBD applications was administered to the BBD group. Among the 29 wounds that were completely debrided at 2 weeks in the BBD group, the mean number of BBD applications applied to each patient was only 3.6 (1.8). The mean number of CO applications administered to the CO group was 19 (4; range, 15–23).
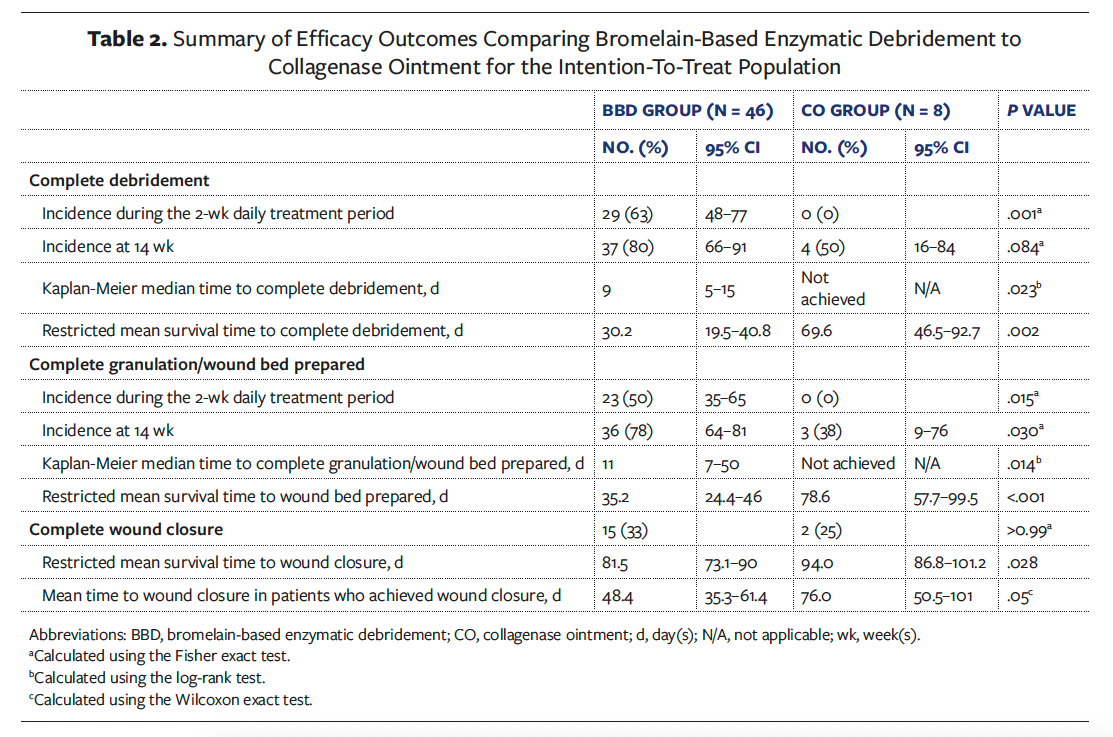
Figure 1 depicts the Kaplan-Meier plot of time to complete debridement. Only the BBD group had a median time to complete debridement (ITT and mITT, 9 days; 95% CI, 5–15) because less than 50% of wounds achieved the event in the CO group and thus, medians could not be calculated (ITT log-rank test, P = .023; mITT log-rank test, P = .019). The RMST to complete debridement was 30.2 days (95% CI, 19.5–40.8) in the BBD group and 69.6 days (95% CI, 46.5–92.7) in the CO group (P = .002). For the mITT analysis, the RMST to complete debridement was 29.7 days (95% CI, 19.2–40.2) in the BBD group and 69.6 days (95% CI, 46.5–92.7) in the CO group (P = .002).
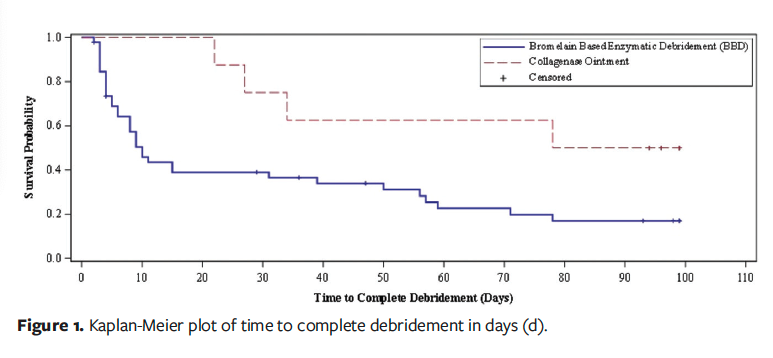
Data for the time to complete granulation and complete wound bed preparation were the same. Figure 2 depicts the Kaplan-Meier plot of time to complete granulation. Median time to complete granulation could only be calculated for the BBD group (ITT: 11 days, 95% CI, 7–50; mITT: 10, 95% CI, 7–39) because less than 50% of wounds achieved the event in the CO group (ITT log-rank test, P = .014; mITT log-rank test, P = .010). The RMST to complete granulation was 35.2 days (95% CI, 24.4–46) in the BBD group and 78.6 days (95% CI, 57.7–99.5) in the CO group (P < .001). For the mITT analysis, the RMST to complete granulation was 33.7 days (95% CI, 23–44.4) in the BBD group and 78.6 days (95% CI, 57.7–99.5) in the CO group (P < .001).
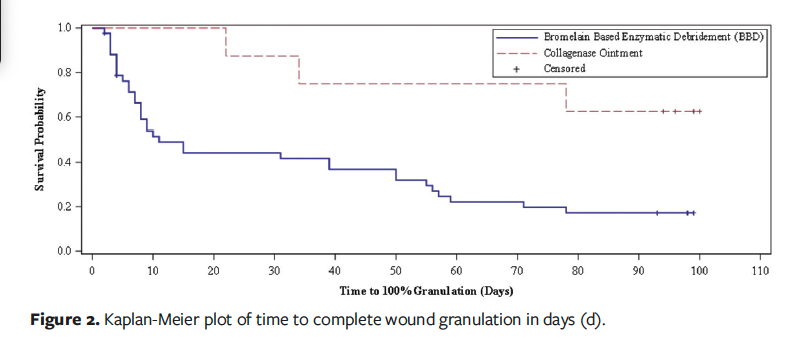
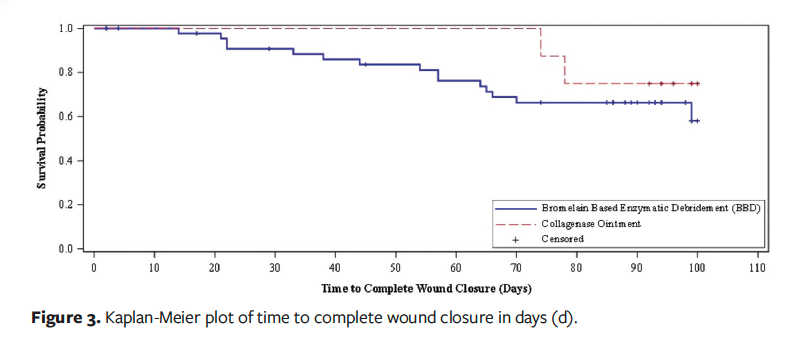
Figure 3 depicts the Kaplan-Meier plot of time to complete closure. The median time to complete closure was not achieved in either group. The RMST to complete closure was 81.5 days (95% CI, 73.1–90) in the BBD group and 94.0 days (95% CI, 86.8–101.2) in the CO group (P = .028). For the mITT analysis, the RMST to complete closure was 80.7 days (95% CI, 72.1–89.4) in the BBD group and 94.0 days (95% CI, 86.8–101.2) in the CO group (P = .021). In patients who achieved wound closure, the average time to wound closure was 48.4 days (95% CI, 35.3–61.4) with BBD vs 76.0 days (95% CI, 50.5–101) with CO (P = .05).
At any time during the study, increased superficial bacterial burden, as assessed using the NERDS criteria, was reported in 67% of subjects in the BBD group (31 of 46) compared with 88% (7 of 8) in the CO group. Deep wound infection, as assessed anytime during the study using the STONES criteria, was noted in 11% of subjects in the BBD group (5 of 46) and in 38% (3 of 8) in the CO group. The AE data in the BBD group have been previously reported in detail.12 Twenty subjects (43%) experienced at least 1 wound-related AE. AEs ≥5% included skin exfoliation (n = 4 [9%]), skin maceration (n = 4 [9%]), wound infection (n = 5 [11%]), and cellulitis (n = 3 [7%]). Similarly, 3 subjects in the CO group (38%) experienced at least 1 wound-related AE, whether wound infection (n = 1 [12%]) or cellulitis (n = 2 [25%]).
Pain levels related to treatment applications were quite similar between groups, with the CO group reporting slightly higher pain levels (Figure 4). Among the 3 subjects with AEs in the CO group, 1 (12%) also reported pain. One patient in the BBD group (2%) discontinued treatment due to pain. During this study, 7 subjects in the BBD group (15%) underwent grafting compared with 1 (12%) in the CO group. Five subjects in the BBD group (11%) received autografts, compared with none in the CO group. Two subjects in the BBD group (4%) received allografts/xenografts, compared with 1 (12%) in the CO group.
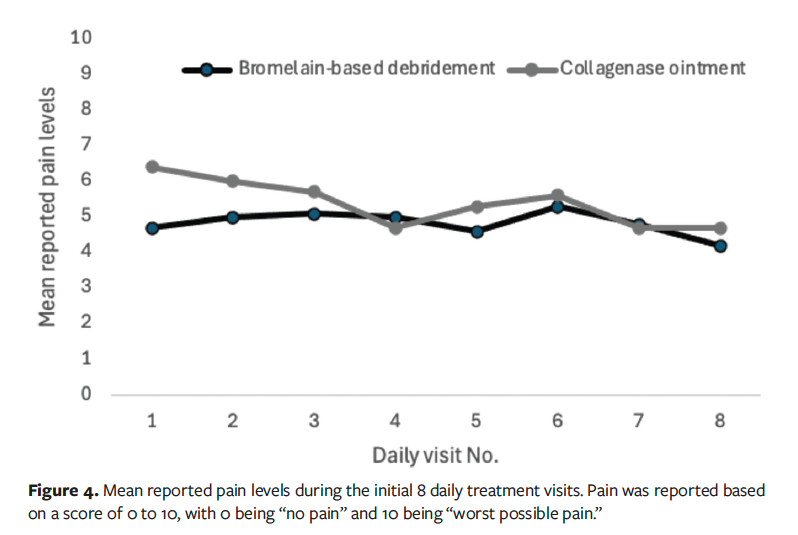
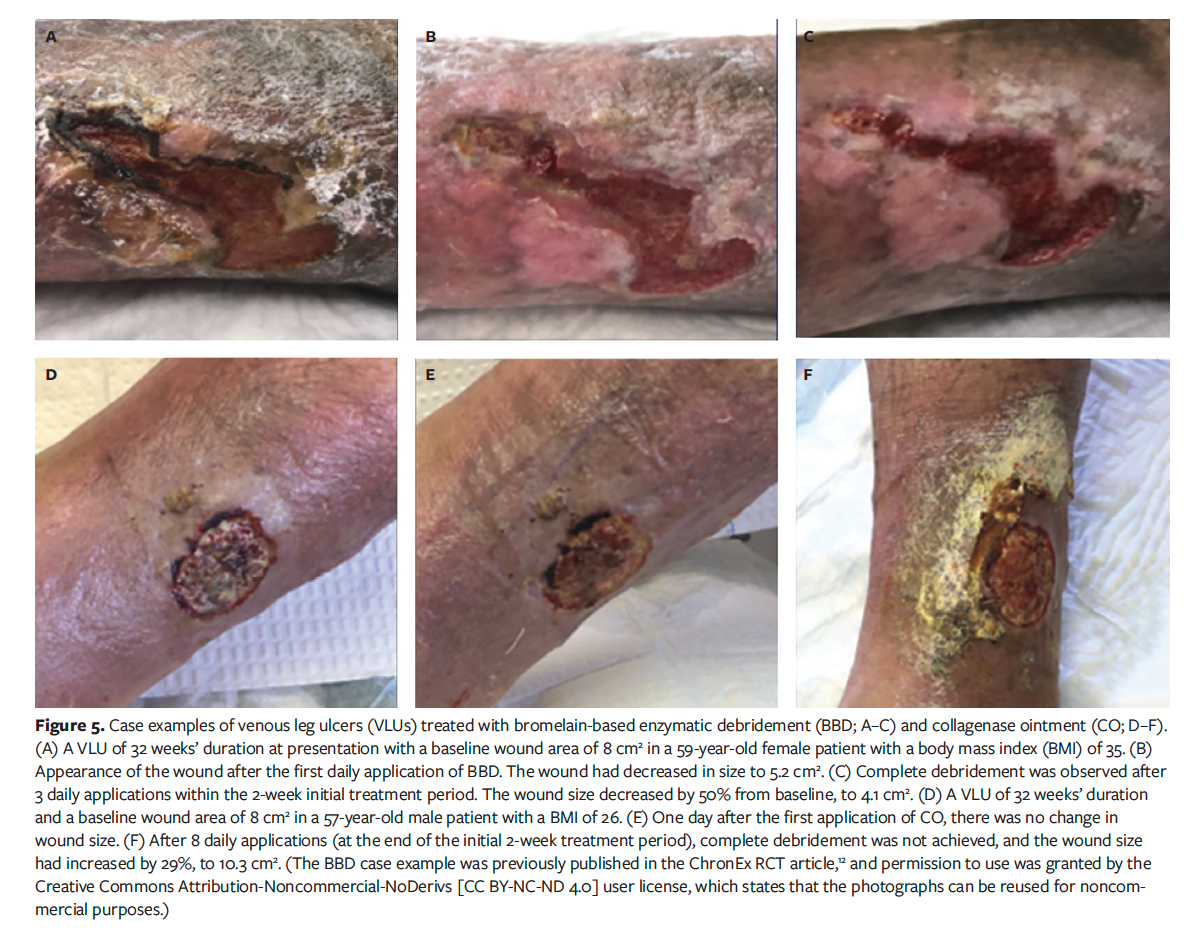
Figure 5 depicts 2 study wounds that at baseline were similar in severity, showing both wounds through the end of the initial 2-week treatment period. One case was treated with BBD, and the other was treated with CO.
Discussion
In this post hoc analysis of the ChronEx RCT, BBD treatment resulted in superior and significantly faster incidence of complete debridement, complete granulation, complete wound bed preparation, and complete closure compared with CO. The post hoc analysis did not compare CO performance with GV, which was evaluated in 43 patients in the ChronEx trial.12 The safety profiles, in terms of pain levels and AEs, of all 3 treatments were similar. However, it is interesting to note that while 63% of BBD-treated wounds and 30% of GV-treated wounds were completely debrided by 2 weeks,12 none of the wounds in the CO group achieved complete debridement at 2 weeks.
In this post hoc analysis, the RMST to complete debridement was just over 4 weeks in the BBD group (30 days), while it was significantly longer (69.6 days) in the CO group (P = .002). Similarly, the RMST to complete granulation/wound bed preparation was approximately 5 weeks in the BBD group (35.2 days) and nearly 10 weeks in the CO group (78.6 days; P < .001). In this post hoc analysis, BBD monotherapy was more effective than CO in preparing the wound bed for healing, with daily treatment required for only 1 week to 2 weeks. Nearly 4 times as many applications were required for the CO group (19 for CO vs 5 for BBD), and the speed at which wound bed preparation was achieved with CO was similar to data previously reported in the literature; in fact, the daily treatment period of CO in clinical trials is usually 4 to 6 weeks, when done in conjunction with sharp debridement.7,11 In one study, CO was mostly applied once daily during the initial 2-week daily treatment period and then per investigator discretion.12 A meta-analysis provided an in-depth look at the effect of CO on DFUs by analyzing 174 patients in 4 small RCTs.7 None of the 4 trials included debridement rates as a primary end point, so it is difficult to compare those data with the data reported in the present post hoc analysis. CO provided therapeutic benefit when applied after an initial sharp debridement and was more effective when applied in combination with weekly sharp debridement.7 Individual clinical trials with CO were not designed to show statistically significant superiority over a comparator.
In the ChronEx trial, the median time to complete debridement for BBD was 9 days; for the GV group, the median time was 63 days.12 Because the CO group had so few wounds achieving complete debridement, a median time could not be calculated. Thus, it would appear that while BBD was the most effective treatment in the ChronEx trial, the GV appeared to have demonstrated better outcomes when compared with CO.12 In an underpowered RCT evaluating CO compared with hydrogel in 215 DFUs, equivalent wound bed preparation outcomes were achieved in both groups; 79% of DFUs in both groups were well-granulated by 12 weeks, and both groups underwent 0.8 sharp debridements per week.23
In a small RCT comparing the effect of 4 weeks of treatment with CO vs SMG on 48 DFUs, both groups had significantly better wound assessment scores compared to baseline (which evaluated debridement and granulation) after 4 weeks of treatment (CO: −2.5, P = .007; SMG: −3.4, P = .006).6 However, there were no significant differences between groups in any wound assessment subscales, including debridement and granulation rates. Similar results were obtained in a small, underpowered trial in which CO plus sharp debridement for 6 weeks was compared with sharp debridement alone for 6 weeks, with improved wound status scores reported in both groups compared to baseline; however, there were no statistically significant intergroup differences.24
Limitations
The ChronEx study limitations were previously reported in detail.12 The data reported herein are based on a post hoc analysis of a prospective RCT, which compared the BBD group with a subset of patients randomized to NSSOC and treated with CO. A specific limitation of the present post hoc analysis is that the CO population sample was very small; however, the group differences were substantial enough to detect statistical significance. It is also notable from the paucity of data in the literature that the use of collagenase in chronic VLUs has not been frequently studied. As previously reported,12 the RCT was double-masked for both BBD and GV groups. However, the NSSOC options clearly differed in appearance and application from the other treatments; therefore, CO and NSSOC treatments were not masked. This may have created bias in the investigators’ assessments. Per protocol, BBD was allowed to be used only during the daily treatment period (up to 8 applications within 2 weeks), while CO could be used throughout the study (up to 14 weeks).
Conclusion
Post hoc analysis from the ChronEx RCT demonstrates a clinically meaningful and statistically significant reduction in time to debridement and a significant increase in incidence of wound bed preparation, including complete debridement and complete granulation, in chronic VLUs treated with BBD compared with those treated with CO. Overall, 3 phase II trials have reported BBD to be safe, well-tolerated, efficacious, and efficient in the debridement and promotion of granulation tissue in hard-to-heal wounds, with only a few daily applications required. BBD appears to be more effective than CO, with a similar safety profile. Phase III evaluation of BBD in patients with VLUs is underway.
Author and Public Information
Authors: Cyaandi R. Dove, DPM1; Robert J. Snyder, DPM, MBA, MSc2,3; Keren David Zarbiv, MSc2; Yael Katz Levy, PhD2; Asi Haviv, DMD2; Ety Klinger, PhD, MBA2; Yaron Shoham, MD4; and Felix Sigal, DPM5
Affiliations: 1University of Texas Health Science Center at San Antonio, San Antonio, TX, United States; 2MediWound, Ltd, Yavne, Israel; 3Barry University, Miami, FL, United States; 4Soroka University Medical Center, Beer Sheba, Israel; 5Angel City Research, Los Angeles, CA, United States
Author Contributions: Study design and protocol development: K.D.Z., Y.K.L., Y.S., and E.K. Data collection: C.R.D., Y.K.L., and F.S. Data curation: K.D.Z. and Y.K.L. Review and/or drafting of manuscript: All authors.
Disclosure: This study was funded by MediWound, Ltd (Yavne, Israel). C.R.D. is a consultant to MediWound. R.J.S. is Chief Medical Officer of MediWound. K.D.Z. is VP of Clinical Affairs of MediWound. Y.K.L. is Director of Clinical Development of MediWound. A.H. is Medical Director of MediWound. E.K. is Chief Research and Development Officer of MediWound. Y.S. is a consultant to MediWound. F.S. discloses no financial or other conflicts of interest.
Ethical Approval: The study adhered to the 1975 Declaration of Helsinki, and the institutional review boards and ethics committees of all participating institutions approved the study protocol.
Correspondence: Cyaandi R. Dove, DPM; Department of Orthopaedics, Division of Podiatry, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, MC 7776, San Antonio, TX 78229-3900; dovec@uthscsa.edu
Manuscript Accepted: January 17, 2025
References
1. Heitzmann W, Fuchs PC, Schiefer JL. Historical perspectives on the development of current standards of care for enzymatic debridement. Medicina (Kaunas). 2020;56(12):706. doi:10.3390/medicina56120706
2. Hu Y, Webb E, Singh J, et al. Rapid determination of substrate specificity of Clostridium histolyticum beta-collagenase using an immobilized peptide library. J Biol Chem. 2002;277(10):8366-8371. doi:10.1074/jbc.M111042200
3. Smith RG. Enzymatic debriding agents: an evaluation of the medical literature. Ostomy Wound Manage. 2008;54(8):16-34.
4. Shi L, Carson D. Collagenase Santyl ointment: a selective agent for wound debridement. J Wound Ostomy Continence Nurs. 2009;36(6 Suppl):S12-S16. doi:10.1097/WON.0b013e3181bfdd1a
5. McCallon SK, Weir D, Lantis JC 2nd. Optimizing wound bed preparation with collagenase enzymatic debridement. J Am Coll Clin Wound Spec. 2015;6(1-2):14-23. doi:10.1016/j.jccw.2015.08.003
6. Tallis A, Motley TA, Wunderlich RP, et al. Clinical and economic assessment of diabetic foot ulcer debridement with collagenase: results of a randomized controlled study. Clin Ther. 2013;35(11):1805-1820. doi:10.1016/j.clinthera.2013.09.013
7. Lantis JC 2nd, Gordon I. Clostridial collagenase for the management of diabetic foot ulcers: results of four randomized controlled trials. Wounds. 2017;29(10):297-305. doi:10.25270/wnds/2017.10.297305
8. Riley KN, Herman IM. Collagenase promotes the cellular responses to injury and wound healing in vivo. J Burns Wounds. 2005;4:e8.
9. Shi L, Ermis R, Garcia A, Telgenhoff D, Aust D. Degradation of human collagen isoforms by Clostridium collagenase and the effects of degradation products on cell migration. Int Wound J. 2010;7(2):87-95. doi:10.1111/j.1742-481X.2010.00659.x
10. Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997;137(6):1445-1457. doi:10.1083/jcb.137.6.1445
11. Patry J, Blanchette V. Enzymatic debridement with collagenase in wounds and ulcers: a systematic review and meta-analysis. Int Wound J. 2017;14(6):1055-1065. doi:10.1111/iwj.12760
12. Shoham Y, Snyder RJ, Katz Levy Y, et al. Once daily bromelain-based enzymatic debridement of venous leg ulcers versus gel vehicle (placebo) and non-surgical standard of care: a three-arm multicenter, double-blinded, randomized controlled trial. EClinicalMedicine. 2024;75:102750. doi:10.1016/j.eclinm.2024.102750
13. Shoham Y, Krieger Y, Tamir E, et al. Bromelain-based enzymatic debridement of chronic wounds: a preliminary report. Int Wound J. 2018;15(5):769-775. doi:10.1111/iwj.12925
14. Rosenberg L, Krieger Y, Bogdanov-Berezovski A, Silberstein E, Shoham Y, Singer AJ. A novel rapid and selective enzymatic debridement agent for burn wound management: a multicenter RCT. Burns. 2014;40(3):466-474. doi:10.1016/j.burns.2013.08.013
15. Shoham Y, Gasteratos K, Singer AJ, Krieger Y, Silberstein E, Goverman J. Bromelain-based enzymatic burn debridement: a systematic review of clinical studies on patient safety, efficacy, and long-term outcomes. Int Wound J. 2023;20(10):4364-4383. doi:10.1111/iwj.14308
16. Singer AJ, Toussaint J, Chung WT, et al. Development of a contaminated ischemic porcine wound model and the evaluation of bromelain-based enzymatic debridement. Burns. 2018;44(4):896-904. doi:10.1016/j.burns.2017.07.022
17. Singer AJ, Goradia EN, Grandfield S, et al. A comparison of topical agents for eschar removal in a porcine model: bromelain-enriched vs traditional collagenase agents. J Burn Care Res. 2023;44(2):408-413. doi:10.1093/jbcr/irac080
18. Shoham Y, Sabbag I, Singer AJ. Development of a porcine hard-to-heal wound model: evaluation of a bromelain-based enzymatic debriding agent. J Wound Care. 2021;30(Suppl 9a):VI. doi:10.12968/jowc.2021.30.Sup9a.VI
19. Shoham Y, Shapira E, Haik J, et al. Bromelain-based enzymatic debridement of chronic wounds: results of a multicenter randomized controlled trial. Wound Repair Regen. 2021;29(6):899-907. doi:10.1111/wrr.12958
20. Snyder RJ, Singer AJ, Dove CR, et al. An open-label, proof-of-concept study assessing the effects of bromelain-based enzymatic debridement on biofilm and microbial loads in patients with venous leg ulcers and diabetic foot ulcers. Wounds. 2023;35(12):E414-E419. doi:10.25270/wnds/23099
21. Sibbald RG, Woo K, Ayello EA. Increased bacterial burden and infection: the story of NERDS and STONES. Adv Skin Wound Care. 2006;19(8):447-463. doi:10.1097/00129334-200610000-00012
22. Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(Suppl 1):S1-S28. doi:10.1046/j.1524-475x.11.s2.1.x
23. Jimenez JC, Agnew PS, Mayer P, et al. Enzymatic debridement of chronic nonischemic diabetic foot ulcers: results of a randomized controlled trial. Wounds. 2017;29(5):133-139.
24. Motley TA, Lange DL, Dickerson JE Jr, Slade HB. Clinical outcomes associated with serial sharp debridement of diabetic foot ulcers with and without clostridial collagenase ointment. Wounds. 2014;26(3):57-64.
















