Advances in Burn Wound Management: Innovative Strategies for Healing and Infection Control
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Burn wounds are insults to the skin that can be caused by various sources, including thermal, electrical, or chemical sources, and even natural sources such as the sun. A burn wound is conventionally categorized into 3 distinct zones: (1) coagulation, (2) ischemia/stasis, and (3) hyperemia. In addition to the potential for physiological scarring, burn wounds can lead to microbial infections, such as pneumonia and methicillin-resistant Staphylococcus aureus, that are difficult to treat using conventional antimicrobial therapy. Patients whose burn wounds trigger a systemic inflammatory response experience further deterioration of their medical condition. Moreover, an increase in the incidence of antibiotic resistance poses a major challenge in the treatment of wounds. Researchers are shifting their focus to newer techniques, such as acellular fish skin, hydrogels, negative pressure wound therapy, nanotherapeutics, and stem cell therapy to counter the disadvantages associated with conventional therapy. This review provides an overview of burn wound causes, classifications, and treatments, and it discusses the healing phases of wounds, possible types of infections, the complexities associated with existing conventional treatments, and the advanced techniques currently used in burn wound management that have proven to reduce hospital stays and make treatment more cost-effective.
Abbreviations: ADSC, adipose tissue-derived stem cell; AFS, acellular fish skin; BMSC, bone marrow-derived stem cell; DPTB, deep partial-thickness burn; ECM, extracellular matrix; IL, interleukin; MRSA, methicillin-resistant S aureus; NPWT, negative pressure wound therapy; P aeruginosa, Pseudomonas aeruginosa; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; S aureus, Staphylococcus aureus; siRNA, short interfering RNA; SIRS, systemic inflammatory response syndrome; SPTB, superficial partial-thickness burn; TBSA, total body surface area; TNF-α, tumor necrosis factor α; UDSC, umbilical cord-derived stem cells.
Burn wounds are insults to the skin and the underlying tissues that are caused by heat, friction, overexposure to the sun, radiation, and chemical or electrical sources. The majority of burn wounds are caused by fire and hot liquids.1 Burn wounds are classified as open wounds, a class that also includes bite wounds, puncture wounds, crush wounds, lacerations, avulsions, and sharp cuts.2 All these wounds have unique pathophysiology and are different from each other. Burn wounds are further classified depending on the causative agent and the extent to which damage occurs. Depending on the causative agent or agents, burns are categorized as physical, thermal, electrical, radiation, laser, or chemical. Depending on the severity, a burn wound can be classified based on the depth and size of the impact site.
Burns that involve the uppermost layer of the skin were formerly classified as first-
degree burns. What were formerly classified as second- and third-degree burns involve the epidermis and extend beyond to the deeper layers, respectively. Presently, burn types are renamed according to severity and depth.1 Superficial burns, also known as first-degree burns, are characterized by redness at the site and limited pain duration. SPTBs, or second-degree burns (formerly known as 2A burns), are painful and may require wound care and dressing.1 DPTBs, formerly known as class 2B burns, involve scarring and are dry. Interestingly, these wounds are less painful owing to the destruction of some of the pain receptors associated with the impact site; such wounds require surgery.1 Third-degree burns are currently known as full-thickness burns, and these involve the full thickness of the skin along with the subcutaneous structures. There is minimal pain because the nerve endings become damaged, and such burns are prone to infection. Fourth-degree burns involve charred skin with injuries to muscle and/or bone.1 Fifth- and sixth-
degree burns are more severe, with exposed bone.3 The normal burn wound healing process is shown in Figure 1.
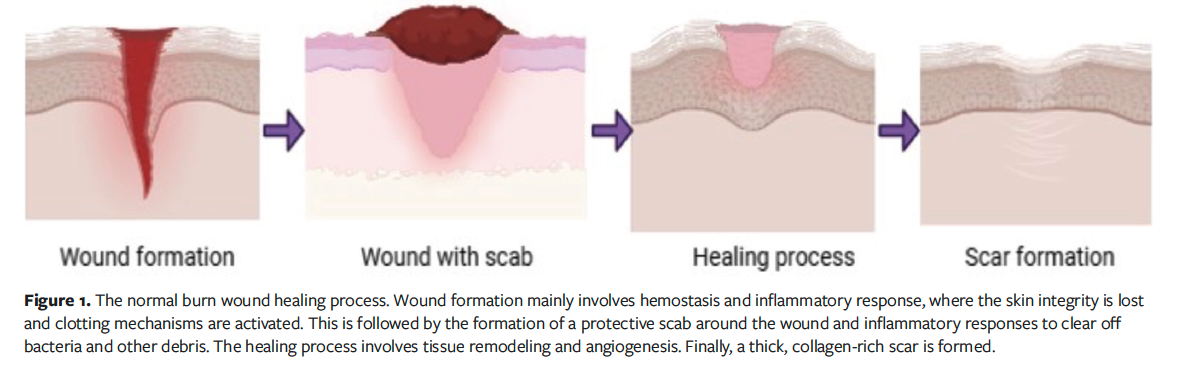
Burns are also classified as major or minor, depending on the extent to which the TBSA is affected by the burn. A minor burn is one that affects less than 10% of the TBSA regardless of patient age; severe burn is defined based on TBSA and patient age, that is, greater than 10% TBSA in geriatric patients, greater than 20% in adult patients, and greater than 30% in children.1
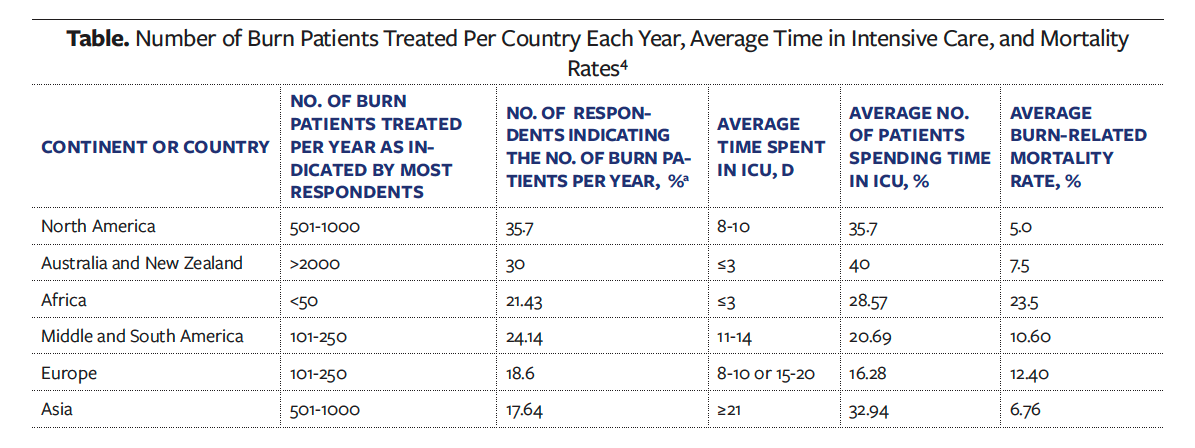
A review published in 2023 estimated the number of burn patients per year to be 101 to 205 per hospital on a global level.4 Approximately 64% of the patients included in that review were adults, with 56.38% being male patients. The mean duration of hospital stay was 11 days to 14 days, and the average time spent in the intensive care unit was 3 or more weeks.4
The number of burn patients treated per country or continent each year, along with average time spent in the intensive care unit and mortality rates, are listed in the Table.4
The present review discusses burn wounds, their types and pathophysiology, the stages in the healing process, the risk of infection, and the advanced techniques more recently being used in the management of burn wounds.
Pathophysiology of Burn Wounds
Burn injury leads to many changes both locally and systemically. Immediately after a burn injury, inflammatory stress responses are triggered wherein an upsurge in the levels of cytokines and chemokines driven by sympathetic tone is observed.1 The extent of damage and the inflammatory response depends on the energy carried by the causative agent, the duration of impact, the TBSA involved, other underlying conditions, patient’s age, and the temperature to which the skin is exposed.1,5 The inflammatory response is triggered mainly to initiate tissue repair and wound healing. However, severe burns may cause dysregulated inflammatory responses whereby the inflammatory cascade may be triggered several times after the initial resuscitation.1,6 This can lead to organ damage and, finally, death.
The burn wound is conventionally categorized into 3 distinct zones: the zone of coagulation, the zone of ischemia/stasis, and the zone of hyperemia.7 The zone of coagulation is the point where maximum damage occurs. This is the point where the constituent proteins coagulate, resulting in irreversible tissue loss. In the surrounding zone of ischemia/stasis there is decreased tissue perfusion; this part of the tissue is usually salvageable. Any other insults, such as infections, hypotension, or edema, can turn this site into an area of complete tissue loss. The zone of hyperemia is characterized by an increase in blood supply due to inflammatory vasodilation in the outermost regions of the wound.7 The extent of damage to cells varies depending on the area affected and can range from instant cellular self-destruction within the initial 24 hours following the injury to apoptosis that occurs later, approximately 24 hours to 48 hours after the burn.1 Reversible oxidative stress is also present.1
Once the burn injury exceeds 30% of TBSA, the inflammatory response at the site of injury triggers a systemic effect, resulting in hypovolemia along with the release of inflammatory mediators with subsequent systemic effects, mainly cardiovascular dysfunction, also known as burn shock.8-10 This condition is irreversible, even with adequate fluid support and proper interventions.10 Other systemic effects include respiratory, metabolic, and immunological changes.7
Burn shock is a combination of hypovolemic, distributive, and cardiogenic shock. In the initial stages, burns cause capillary leakage due to an increase in capillary permeability.11 This leads to the loss of intravascular proteins and fluids into the interstitial compartments, further resulting in reduced cardiac output, and hypovolemia requiring fluid resuscitation.11,12 Myocardial contractility and cardiac output are reduced, possibly due to circulating inflammatory mediators, such as TNF-α, that impair calcium transport in the myocardium.7,11
Edema formation is another response to burn injury. Edema formation is a biphasic response consisting of a primary phase followed by a secondary phase. The primary phase starts during the first few minutes after injury, when there is a quick rise in the water content of the affected tissue.10 The secondary phase begins after 8 to 12 hours and lasts up to 24 hours after the burn injury; this phase involves a slow accumulation of fluid in both the burnt and unburnt skin.10,13 Respiratory changes include bronchoconstriction due to inflammatory mediators.7
The type of fluid resuscitation administered plays an important role in influencing the rise in tissue water content during postburn edema. Within the first hour, the water content of the tissue doubles its original volume. Boosting blood flow and capillary pressure is essential for effective fluid resuscitation, helping to minimize further fluid leakage. Plus, edema usually stabilizes when fluids are carefully managed.14,15
Severe burns can also cause a hypermetabolic response that can last up to 2 years postburn. The hypermetabolic response is characterized by a 3-fold increase in metabolic rate, slowed growth, multiple organ dysfunction, insulin resistance, and increased risk for infection.16-20 There is also an upsurge in catecholamines levels in plasma that can last up to 9 months postburn.21 A rise in the levels of IL-1, IL-6, TNF-α, platelet-activating factor, dopamine, cortisol, glucagon, complement cascades, and ROS has also been observed.22
Upregulation of adaptive immunity and adaptive biomarkers, and downregulation of innate immunity, was observed in a recent study.23 An impaired immune response can lead to sepsis due to increased susceptibility to infections, augmenting systemic inflammation.23 Persistent hypermetabolism coupled with inflammation slows reepithelialization and impairs the wound healing process.24,25 However, the extent of hypermetabolism and systemic inflammation depends on the burn depth because the higher the impact, the higher the circulating cytokines, with resultant greater hypermetabolic responses.26
Thermal injuries include scalds, exposure to flame, and often direct contact with substances capable of causing burns.7 It has been observed that thermal insults, in addition to impairing the normal functioning of a cell at the impact site, also adversely affect regions like the skeletal muscles, which are distant from the injury site. Studies have shown that the membrane potential of the impact site and the intact skeletal muscle distant from the injury site demonstrates partial depolarization and changes the potential from −90 mV to −80 mV and −70 mV.5 A decline in the membrane potential results in an increase in sodium and water molecules within the cells, an environment similar to hemorrhagic shock. Similar results have been observed in other cells, including endothelial, cardiac, and hepatic cells.27
Burn Wound Healing
One of the major goals in burn wound healing is speedy restoration of healthy skin with minimal scarring. Wound healing is a complex phenomenon that involves cross talk among the vascular system and cytokines and inflammatory mediators. The overall healing process consists of 4 overlapping phases: (1) hemostasis, (2) inflammatory, (3) proliferation, and (4) remodeling.28,29 The hemostasis phase is characterized by platelet aggregation, immune system activation, blood clotting, and activation of the complement cascade.30 The inflammation phase involves the production of neutrophils and macrophages by the resident cells in the skin. The proliferation phase involves reepithelialization, angiogenesis, and granulation. The collagen phase involves production and maturation of collagen, elastin, and fibroblasts.30
Hemostasis phase
The hemostasis phase occurs within 10 minutes of the thermal insult and is characterized by vasoconstriction and clot formation. This phase is the result of the autonomic response, which is triggered in an attempt to reduce the damage. As discussed above, this phase includes platelet aggregation, activation of the immune system, blood clotting, and activation of the complement cascade.31 The formed clot and the impacted tissue release a set of inflammation-promoting cytokines and various growth factors for healing.32
Inflammatory phase
The inflammatory phase starts within 3 days after the insult and involves hemostasis and chemotaxis. This phase is classified into an early and a late phase. In the early phase, neutrophils arrive at the site of injury, promoting inflammatory response by producing inflammatory mediators such as IL-1, IL-6, and TNF-α. In the late phase (3 days after the insult), the monocytes mature to become macrophages.33 The PDGFs attract fibroblasts and promote their multiplication, which eventually synthesizes collagen. In addition to the action of PDGFs, other factors contribute to revascularization, reepithelialization, fibroblast transformation, and collagen degradation. Autacoids, such as serotonin and histamine, enhance cellular permeability.34
Proliferation phase
The proliferation phase involves a few other processes, such as reepithelialization, angiogenesis, and the formation of granulation tissue. This phase is active from day 3 to day 10,31 during which time the fibroblasts lay down collagens and glycosaminoglycans, which help stabilize the wound. The reepithelialization phase involves the expansion of stem cells, keratinocytes, fibroblasts, and epithelial cells. In the process of restoration during burn injury, migration and proliferation occur.31 Keratinocytes, fibroblasts, and fibroblast-derived macrophages play a crucial role in this process. Additionally, some fibroblasts differentiate into myofibroblasts, which are instrumental in the production of collagen, leading to the formation of mature scars.31,35 Moreover, in this phase, neovascularization occurs as a result of angiogenesis, and vasculogenesis happens, which is the formation of new vessels from epithelial progenitor cells.36 As discussed above in the “Inflammatory phase” section, growth factors such as transforming growth factor β, fibroblast growth factor, epidermal growth factor, PDGF, and granulocyte-macrophage colony-stimulating factor (GM-CSF) are involved in activating endothelial cell growth. Following activation, the endothelial cells further secrete proteolytic enzymes, which initiates breakdown of the basal lamina, creating a conducive environment that facilitates their proliferation and migration into the wound.31 In the latter part of the proliferation phase, the emergence of granulation tissue is indicated by the presence of granulocytes, fibroblasts, and macrophages. Fibroblasts, which play a crucial role in the granulation phase, generate ECM and collagens. ECM plays a crucial role in supporting cell adhesion and contributing to the growth and differentiation of cells such as fibroblasts. In the final stages of this phase, fibroblasts differentiate into myofibroblasts, which later form a scar that undergoes programmed cell death.37,38
Remodeling phase
The remodeling phase is the last step in wound healing. It starts approximately 2 weeks to 3 weeks after the insult and continues for 1 year or longer.30 In this phase, an increased amount of elastin and collagen is produced, and maturation of fibroblast to myofibroblast occurs.
Infections in Burn Wound
Infections are a major threat to patients after burn wound injury. Loss of skin barrier provides a favorable environment for microorganisms to colonize and grow.39 A few risk factors that facilitate microbial colonization and infections are burn wound depth, patient age, and impaired immunity. Bacteria and fungi are the most common pathogens that colonize burn wound.40 The most common gram-positive bacteria involved in infection include Staphylococcus spp, Streptococcus pyogenes and Viridans Streptococci, and Enterococcus spp, with Staphylococcus spp being the major culprit.41-44 Gram-negative bacteria include Klebsiella spp, P aeruginosa, Acinetobacter baumannii, Escherichia coli, Stenotrophomonas spp, and Enterobacter cloacae.39,41,42,45 Apart from gram-positive and gram-negative bacteria, multidrug-resistant organisms such as MRSA, vancomycin-resistant Enterococcus, multidrug-resistant Pseudomonas spp, and Acinetobacter can lead to infections in patients with severe burn wounds.41 These infections can lead to prolonged hospital stay with higher mortality in burn patients than in patients without burn injury.44 It has been observed that both skin and soft tissues become infected earlier during hospitalization, while bloodstream infections, pneumonia, and urinary tract infections occur at later stages of hospitalization. The median onset of the late-stage infections has been found to be greater than 30 days after hospitalization.46
Complexities in conventional antimicrobial therapy
A multitude of complexities arise from conventional antimicrobial therapy in patients with burn injuries. It can be challenging to identify the infection, which in turn can make it difficult to select an effective antimicrobial. Critically ill burn patients may experience SIRS, which can be mistaken for an infection. Symptoms of SIRS include fever, increased heart rate, and elevated inflammatory markers. These symptoms are also present during the sepsis response. SIRS and sepsis also elevate C-reactive protein and procalcitonin levels via bacterial infection and tissue injury. Omics technology, which involves metabolomics, proteomics, and transcriptomics, is being investigated as an option to enhance the identification of sepsis in patients with burn injuries.47
Another problem is the intricate microbiology of burn wounds. Microorganisms such as Haemophilus influenzae and S aureus are generally cultured from respiratory samples taken from patients with inhalational injury. In light of the intricate microbiological nature of burn wounds, coupled with the occurrence of severe sepsis, patients are often treated with broad-spectrum antibiotics. Once the results of the cultures are available, antimicrobial therapy targeting the specific microorganisms is begun. Because patients with burn injury have a lengthy duration of hospital stay, they are at increased risk of nosocomial infection. As a result, exposure to broad-spectrum antibiotics must be limited, because the patients tend to develop antibiotic resistance.48
The presence of SIRS, sepsis, and fluid imbalances may augment the renal clearance of conventional antimicrobials, such as β-lactam antibiotics and vancomycin, which can lead to changes in volume of distribution and insufficient drug exposure to the required compartment.47 Therefore, optimal dosing of antimicrobials in burn patients is needed. Extremes of age can also alter the pharmacokinetics.47
Acellular Fish Skin
AFS is a popular xenograft used to facilitate wound healing. Two major species, Nile tilapia and North Atlantic cod, have been used in the development of AFS. Nile tilapia, or Oreochromis niloticus, skin is a widely known occlusive dressing for the treatment of burns.49,50 North Atlantic cod, or Gadus morhua, is used worldwide and was approved by the United States Food and Drug Administration for the treatment of various wounds in 2013.51 Unlike mammalian skin, such as bovine skin and porcine skin, AFS does not contain microorganisms such as prions and viruses that can be transmitted to humans.52,53 In fact, fish skin has multiple physical and mechanical properties—the most important of which is its antimicrobial action against pathogens—that make it an alternative for skin regeneration.54 In contrast to mammalian skin, AFS retains its natural structure and collagen, which maintains its mechanical strength and creates an appropriate tissue repair and regeneration microenvironment. The fish skin can be decellularized without complex chemical processes while retaining the structure and composition of biologically active compounds, including omega-3 unsaturated fatty acids.55 Studies have shown that fish skin is rich in omega-3 polyunsaturated fatty acids, docosahexaenoic acids, and eicosapentaenoic acids, which reduce inflammatory responses but promote the pro-inflammatory cytokines that are involved in wound healing.56 AFS rich in omega-3-polyunsaturated fatty acids can facilitate a shift from the inflammatory phase of wound healing.51
The effect of AFS on reepithelialization time has been studied. The reepithelialization time for SPTB is approximately 2 weeks, and for DPTB is more than 3 weeks. Preclinical studies on rabbits57,58 and pigs,59 for example, have shown the potential of AFS in accelerated burn wound healing. The remarkable results achieved in the preclinical studies were taken forward to clinical trials.50,60,61
Silver sulfadiazine 1%, a broad-
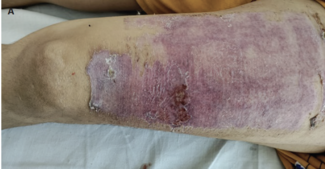
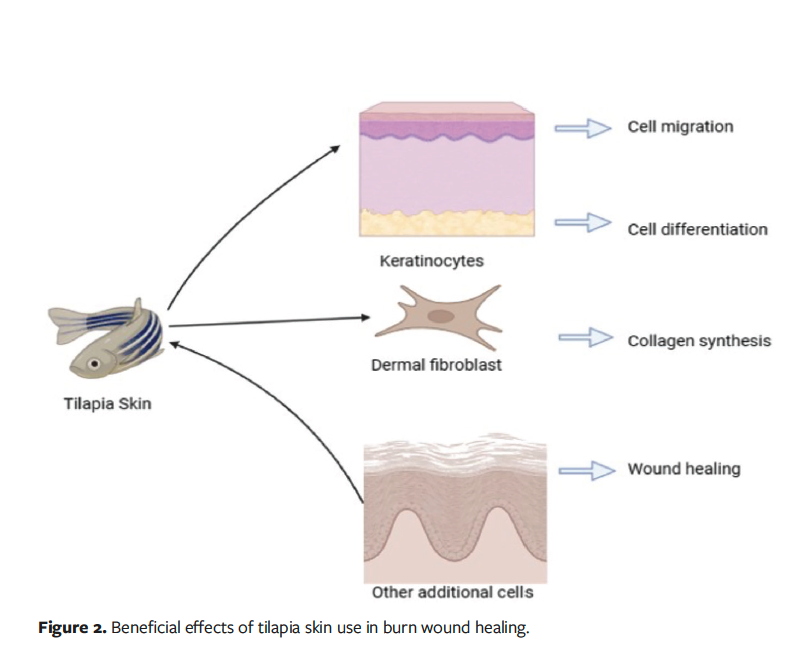
spectrum antibacterial cream, is the standard in the treatment of burns and promotes reepithelialization.62 In a phase 2 randomized controlled trial, silver sulfadiazine was used in the management of SPTB and DPTB.63 The total reepithelialization time for SPTB and DPTB after treatment with silver sulfadiazine was approximately 10 days to 11 days, and 21 days, respectively. In the same study, treatment with Nile tilapia skin achieved complete reepithelialization in a shorter period of time, with an average of 1.43 days less for outpatients and 1.14 days less for inpatients compared with the standard treatment group.63 In addition to reduced reepithelialization time, accelerated wound healing and a 42.1% reduction in total cost per patient were observed in a phase 3 trial involving patients with SPTB, which suggests that it is a viable alternative to conventional treatment.50 Overall, AFS is a novel and potential therapeutic option in the management of burn wounds and offers multiple advantages over conventional methods. The effects of tilapia fish skin on burn wound healing are shown in Figure 2.
Stem Cell Therapy
Over the past several years, researchers have shown interest in stem cell therapy. Several research studies have provided evidence of the effectiveness of stem cells, particularly in the areas of tissue transplantation and oncology. These studies have shown positive results in terms of efficacy.64 Stem cell therapy utilizes the self-renewing and differentiating properties of stem cells to replace damaged cells with new, healthy, functional cells by transplanting exogenous cells into the patient. In 1868, German biologist Ernst Haeckel first described the term “stem cell” as the ability of a fertilized egg to produce all cells of the organism.65
Three types of stem cells are categorized according to their ability to differentiate. Totipotent cells can become any type of cell in the body. Pluripotent cells can become either germline or somatic cells. Multipotent cells can exclusively divide into specific cell types, tissues, or organs. Diverse stem cell sources comprise burn-derived mesenchymal stem cells, hair follicle stem cells, BMSCs, embryonic stem cells, UDSCs, and ADSCs.66 The use of stem cells in the treatment of burn wounds is an area of great interest because they can be used to treat various types of wounds depending on the type of stem cell used.67,68
Stem cells can uniquely regulate the secretion of chemokines, cytokines, and growth factors critical for wound healing. It is also being increasingly reported that the therapeutic actions of stem cells depend on the release of paracrine or signaling molecules.69 Proteomic analysis of BMSCs, ADSCs, and UDSCs shows the release of cytokines, chemokines, and growth factors involved in the migration and proliferation of endothelial cells, keratinocytes, and fibroblasts.69
In an animal study, Shumakov et al70 reported the first application of stem cell therapy, comparing the effects of BMSCs on embryonic fibroblasts in burn wound healing in Wistar rats. The use of allogenic and autogenic fibroblasts-like BMSCs accelerated the wound healing process by enhancing neo-angiogenesis and alleviating inflammation.70 In a human study, allogenic fibroblast-like mesenchymal stem cells were applied on a female patient with 40% TBSA burns.71 Rapid neo-angiogenesis was achieved, along with expedited wound healing and rehabilitation.
In a preclinical study, human umbilical cord mesenchymal stem cells were used on burns in rats.72 Expedited wound healing occurred in the therapeutic group due to decreased levels of IL-1, IL-6, TNF-α, and inflammatory cell infiltrate, and an increase in the ratio of type Ⅰ and III collagen. The same study also confirmed increased neo-angiogenesis on laser flow Doppler.72
Stem cells also have the potential to treat scars. Stem cells inhibit the actions of keloid fibroblast via paracrine signaling, thus proving to be a potential alternative in managing long-term burn injury.66 ADSC-derived medium has shown reduced inflammation and fibrosis in a keloid implantation animal model.73 These results were replicated in another study that involved the use of a BMSC-derived medium.74 All these results show the advantageous performance of stem cells in burn wound care compared with the traditional methods used.
Hydrogels in Burn Wound Healing
Hydrogels are 2-component systems consisting of hydrophilic polymer and water. Because the main component of hydrogel is water, it hydrates the wound, absorbs exudates, and causes autolysis of the devitalized tissues.75 Hydrogels are also immunologically neutral, which makes them suitable for burn wound management.76 They are available in solid forms as well as gels. Hydrogels have been found to accelerate wound healing in various phases, to expedite autolytic wound cleansing, and to reduce pain.77 The most significant characteristic of hydrogels is their water absorption ability, which results from the structure’s cross-linking and the existence of hydrophilic or hydrophobic monomers.78 The cross-linking substance plays a crucial role in determining the properties of hydrogels. Examples of cross-linking substances include ethylene glycol dimethacrylate, or polyethylene glycol diacrylate, N,N'-methylene-bis-acrylamide, and 1,1,1-trimethylolpropane trimethacrylate.78 Hydrolysis is the process by which such substances are degraded. Typically, hydrogel sheets are devoid of any antimicrobial content, but they can be incorporated with active substances or agents such as chlorhexidine or betadine to fight infections.79 In a few studies, hydrogels were incorporated with ionized silver nanoparticles to create a hostile environment for bacterial growth, thus showing that hydrogels can act as vectors for antimicrobials.80-82 Pathogenic growth of Vancomycin-resistant Enterococcus faecalis (VRE), P aeruginosa, and S aureus is greatly inhibited when dressings with this technology are used. Hydrogels incorporated with ionized silver nanoparticles also prevent the development of biofilm on the wound surface.81,83 Studies have shown that approximately 75.9% of silver is released from dressings containing silver during the first 72 hours of application and that these dressings are noncytotoxic.80,84 However, there are few studies that also showed the inability of hydrogel dressings to inhibit the incidence of P aeruginosa infections requiring intravenous antibiotics.83,85,86
Hydrogels also possess antifungal properties.83 Various wound dressings have been developed to facilitate the healing process. Microporous chitosan nano silver/zinc oxide composite dressing reportedly exhibited effective antimicrobial activity.87 Hydrogels containing 1% silver sulfadiazine have been shown to improve wound healing.88 Honey can also be integrated into the hydrogel composition, which can help reduce the release of inflammation-promoting cytokines such as IL-1α, IL-1β, and IL-6.89,90
Chitosan-containing hydrogels are known to enhance the proliferation of fibroblasts, along with type III collagen secretion, and to promote macrophage migration as well as burn wound autolysis.91 Hydrogels also help improve neovascularization.92 Histopathological studies have shown the formation of new vessels and the acceleration of epithelial proliferation.93 Dextran-based hydrogels enhance vascular endothelial growth factor receptor 2 and luminal surface formation to stimulate neovascularization.94,95
Hydrogel dressings with antioxidant properties are being studied because the redox balance in cells plays a major role in managing wounds caused by burns. It is a known fact that at low levels, ROS play a beneficial role in wound healing by promoting the process, whereas increased ROS cause cellular destruction and ultimately impair the wound healing process. Hydrogel dressing with antioxidant properties is known to remove the excess ROS generated from burn wounds, thus reducing oxidative stress and creating a more favorable condition for better wound healing.96 Overall, hydrogels present a secure and effective alternative for burn wound healing and can be used in treating all stages of burn wound healing.
Nanotherapeutics in Burn Wound Healing
In recent decades, nanotherapeutics have been widely used applications of nanotechnology in medicine.97 Nanotherapeutics and nanodiagnostics have been used in the management of a wide range of conditions, including neurological, cardiovascular, dermatological, and inflammatory conditions, as well as cancer.98-102 Nanotherapeutics have a wide range of applications compared with conventional medicine, such as modifying the basic physicochemical properties of conventional therapeutics, increasing the bioavailability of the drug in diseased areas, and reducing the dosage and frequency.103 Nanoparticles such as silver, zinc oxide nanoemulsions, and chitosan nanoparticles have antibacterial activities and can prevent infections arising from burn wounds.104 Furthermore, nanotherapeutics offer additional advantages, such as overcoming bacterial drug resistance, reducing the overall time to wound healing, and high biocompatibility. The biocompatibility and efficacy of nanotherapeutics have been established in both animal models and in humans. However, research in this field continues.30
Due to their aforementioned advantages, polymers, metals, and their oxides have been extensively used in the treatment of burn wounds. Silver, zinc oxide, copper, and gold nanomaterials are the most widely used. These nanomaterials have a broad spectrum of antibacterial activity. Their main actions are generating ROS, breaking down biofilms, and damaging the bacterial DNA to restrict their growth.105,106
A few strategies, like encapsulating antibiotics in polymeric nanomaterials to prevent wound disease and encapsulating growth factors in such nanomaterials, reduce the overall wound healing time.107,108 The antibacterial activity of silver nanoparticles is superior to that of ionic silver because of the capacity of silver nanoparticles to penetrate the cell wall of bacteria.109 As a result, silver nanoparticles are widely used in the management of burn wounds.110-112 The main mechanism of action of zinc oxide is the cleavage of bacterial cell membrane when captured by bacterial cell wall.113,114 In addition, the semiconductor properties of zinc make it possible for zinc oxide nanoparticles to improve cell adhesion, proliferation, and migration through pathways activated by growth factors, thus serving as a sustained source of ionic zinc for the treatment of burn wounds.115 A research study explored the potential of phytochemical-capped gold nanoparticles for therapeutic applications.116 The study involved transdermal drug delivery of these nanoparticles to treat surgically wounded and burnt dorsal skin in mice. In vivo experiments illustrated that using gold nanoparticles expedited the growth efficiency of dorsal skin. The treatment also enhanced both dermal and epidermal thickness, dampened collagenase expression, and induced antioxidants. Additionally, gold nanoparticles were enclosed in Pluronic F-127 (Sigma-Aldrich) and hydroxypropyl methylcellulose to create thermoresponsive gels designed for managing infected burn injury in mice.116 Histopathological studies have confirmed that gold nanoparticles have antibacterial activity and among the highest wound-healing activities. Moreover, gold nanoparticles also reduce oxidative stress and increase the antioxidant defense enzymes within burn wound tissues.117
Nanogels consist of either hydrophilic or lipophilic polymers generated through chemical or physical cross-linking with nanoparticles. In vitro studies have confirmed the antibacterial activity of these nanogels loaded with metallic antimicrobial agents against P aeruginosa, Escherichia coli, S aureus, and MRSA.118 In vivo studies have confirmed that nanogel loaded with antimicrobial agents reduces inflammation, eliminates pathogenic bacteria, and accelerates tissue regeneration.118 Apart from metallic antimicrobial agents, phytomedicines such as curcumin, growth factors, and peptides have also been enclosed within nanogels for the management of burn wounds.119,120
Nanofibers have been used in the management of burn wounds. Certain polymers, such as polyethylene terephthalate, polyacrylic acid, polydimethylsiloxane, polyurethane, and polymethyl methacrylate, have been used in fabricating nanofibers. Antimicrobials such as cefotaxime and plant extracts such as Lawsonia inermis (ie, henna) are loaded into the nanofibers to treat burn wounds infected with bacteria, thus promoting healing.121-128 Heparin-mimetic peptide nanofibers were used to facilitate tissue regeneration in full-thickness burn injuries, mitigating tissue function loss at the wound site.129 Using self-assembling short peptide nanofibers has become a recent approach to accelerate the aesthetic restoration of skin damaged by burns.128
Nanosheets are 2-dimensional nanoparticles with thickness ranging from 1 nm to 100 nm.130,131 Nanosheets have unique features such as increased flexibility, adhesive strength, and increased transparency that make them an alternative for the treatment of burn wounds.132 Nanosheets coat the wound and protect it from the environment, and they provide a visual field for observing recovery of the wound. Nanosheets can be loaded with antimicrobial agents such as zinc oxide and silver nanoparticles,132 siRNA,133 and silk fibroins134 to manage various types of wounds. Silver sulfadiazine-loaded nanosheets showed efficacy against MRSA in vitro, and in vivo studies in mouse models reported significant reductions in MRSA count as well as inflammation in partial-thickness burn wounds.132 Nanosheets loaded with siRNA have been used to silence growth factors so as to reduce cutaneous lesions.133
Nanoemulsions are carrier systems that have thermodynamic stability and are used in burn wound healing. Nanoemulsions can be generated from oils with antimicrobial properties, such as Cleome viscosa oil, clove oil, garlic oil, and cinnamon oil.135-137 The oil in the nanoemulsion fuses with the bacterial membranes, resulting in the destabilization and lysis of pathogens.138 Thakur et al139 showed that fusidic acid loaded into cationic bilayered nanoemulsions prevented bacterial penetration and served as a drug reservoir. These nanoemulsions have advantages including improved drug permeation, accelerated wound contracture, reduced bacterial count, and reepithelialization. These nanoemulsions have the ability to generate thin films over the wound and aid in healing.140 Nanotherapeutics can be a potential alternative to burn wound healing and have been shown to be superior to conventional methods.
Negative Pressure Wound Therapy
NPWT is a nonsurgical technique that uses negative pressure to treat burns of diverse etiologies. NPWT is increasingly being used in many surgical specialties. Due to its ability to accelerate wound healing and reduce hospital stay, NPWT has become a widely accepted method in the management of soft tissue defects. NPWT creates a moist environment around the wound, which helps to reduce swelling and increase blood flow to the wound. This, in turn, promotes the development of granulation tissue and stimulates the growth of new blood vessels, ultimately leading to a reduction in the size of the wound. Although the exact way in which NPWT supports wound healing is not yet fully understood, some researchers believe that it may help remove inflammatory exudate from the donor site and reduce the risk of infection. Moreover, Chen et al141 highlighted the positive effect of wound stiffness on cell migration during healing, in which NPWT plays a pivotal role, thereby speeding up wound healing. In a study of patients with bilateral hand burns, Kamolz et al142 evaluated the effectiveness of NPWT vs traditional silver sulfadiazine dressings with silver sulfadiazine cream. The study included patients who were treated within 6 hours of injury. Notable improvement was found in the treatment group starting from the third day after the injury.142 This improvement correlated with a decrease in edema on clinical examination and a decreased rate of progression to full-thickness injury that required skin grafting. Preclinical and clinical studies have shown reduced bacterial count after 4 days of NPWT.143,144 Moreover, studies have also found that NPWT may reduce inflammation associated with acute and chronic wounds.144,145
NPWT has been recommended as an efficacious method for preparing wound beds for skin graft. Successful skin graft is reliant on many factors, including the quality of the recipient wound bed. In addition, the wound bed needs to have a good blood supply and low levels of bacterial infection. NPWT has also been used as a bolster dressing for autografts,146 reducing hospital stay,146 and also as a dressing for the integration of dermal substitutes.147
Importance of This Study
The importance of advances in burn wound management lies in the continuous improvement of treatment outcomes, patient recovery times, and overall quality of life for patients with burn injury. Cutting-edge techniques, including regenerative therapy, nanotechnology, and sophisticated wound dressings, have greatly accelerated the healing process and reduced side effects such as infection and scarring. Because these developments offer fresh perspectives and approaches that may be used everywhere to enhance burn treatment, they are essential to the corpus of literature on wound care. Additionally, they emphasize the significance of individualized treatment regimens and interdisciplinary approaches, both of which are necessary for successful burn management.
Although burn wound care is a worldwide issue, India has particular difficulties with such care because of the high rate of burn injuries and scarcity of resources in the country. The nation has made great progress in creating accessible and affordable treatment choices, which may provide a fresh viewpoint on burn care in environments with limited resources. Similar inventions may be sparked by the progress shown by the country to make treatment affordable in other areas dealing with comparable difficulties. Advances in burn care in India may inspire additional creative solutions in other countries with limited resources and a high rate of burn injuries.
Burns present unique management challenges due to several factors, including burn severity and complexity, infection risk, fluid loss and metabolic stress, scarring, and contractures. Therefore, research specifically focused on burn wound management is crucial for improving patient outcomes. While the fundamental principles of burn care are universal, certain aspects can be influenced by regional factors, which highlights the value of research from different parts of the world on topics such as the prevalence of burn injuries, access to resources, traditional medicine, and local practices. Research from different geographic regions, including India, can offer valuable insights and perspectives, leading to improved burn care practices globally.
Limitations
This review has a few limitations. First, due to the rapid evolution of the fields of nanotechnology and regenerative medicine, some topics on burn wound healing may have limited research updates. Secondly, most published research focuses on positive treatment outcomes rather than negative outcomes, which may bias the present review to highlight more positive than negative results. Thirdly, most of the published research focuses on short-term outcomes rather than long-term outcomes, thus limiting this review’s ability to draw conclusions about the durability of the treatment. Fourth, this review lacks statistical power because a meta-analysis on this topic was not done.
Conclusion
This review highlights the importance of burn wounds, how burn wounds can lead to harmful infections such as pneumonia and MRSA, and the advanced techniques currently being explored and used to accelerate the wound healing process and reduce the length of hospital stay. The review also addresses the problems of conventional antimicrobial therapy used for wound healing; the emergence of antibiotic resistance, which makes the overall treatment more complex; and the risk of superinfections. In addition to the techniques discussed herein, other advanced techniques are being studied by scientists and used by health care practitioners; these advanced techniques include but are not limited to 3-dimensional bioprinting, hyperbaric oxygen therapy, artificial skin substitutes, and gene therapy, which are beyond the scope of this review.
Author and Public Information
Authors: Alosh Greeny, MPharm; and Rekha R. Shenoy, PhD
Affiliations: Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, India
ORCID: Shenoy, 0000-0001-7684-1242
Disclosure: The authors disclose no financial or other conflicts of interest.
Ethical Approval: This is a review article and did not involve any human participants or animal research; as such, ethical approval was not required.
Correspondence: Rekha R. Shenoy, PhD; rekha.shenoy@manipal.edu
Manuscript Accepted: February 5, 2025