Use of a Novel Zipper Device for Wound Closure of Cutaneous Abscesses in Pediatric Outpatients: A Case Series
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. Current management of pediatric cutaneous abscesses involves either spontaneous healing by secondary intention or suturing through tertiary intention, which are often lengthy processes that cause discomfort and distress among children. As it is noninvasive and simple, a novel zipper device is widely used for the primary wound closure of surgical incisions. Objective. To describe the effectiveness of novel zipper device use for pediatric cutaneous abscess wound closure in an outpatient context. Materials and Methods. A total of 26 pediatric outpatients with simple cutaneous abscesses were included. After incision and drainage (I&D), the novel zipper device was applied once pus decreased significantly and fresh granulation tissue emerged. Wound healing time and pain score were tracked over the intervention period. Linear regression and restricted cubic spline models were also used to analyze the relationship between the intervention interval of time from I&D to zipper application and wound healing time. Results. The average (SD) wound healing time was 12.73 (3.16) days. Pain scores decreased significantly from a median of 2 during zipper use to 0 at removal. Linear regression analysis revealed 2 healing time predictors: infection size (B = 0.260, SE = 0.090, β = 0.260, t = 2.924, P = .008) and intervention interval of zipper use (B = 0.850, SE = 0.090, β = 0.810, t = 9.187, P = .001). A linear dose-response relationship was observed between intervention interval and healing time (Ptotal < .001, Pnonlinear = .406). Conclusion. This case series identifies the fifth day post-I&D as a potential threshold, and suggests that the optimal period of zipper device use is 3 days to 5 days following I&D of skin abscess to enhance early wound healing, beyond which extended intervention intervals prolong wound healing time.
Cutaneous abscesses, purulent skin, and soft tissue infections affecting the skin, dermis, and subcutaneous tissue are frequently managed in pediatric outpatient clinics.1 The primary pathogen in these abscesses, Staphylococcus aureus (S aureus), exhibits methicillin-resistant S aureus (MRSA) in 46% to 77% of cases,1 and the biofilms formed by S aureus further enhance MRSA antibiotic resistance.2-4 This growing multidrug resistance has led to a rise in cutaneous abscess prevalence.3 Once a cutaneous abscess is diagnosed, incision and drainage (I&D) and antimicrobial agents or calcium alginate dressing changes are recommended for treatment.5,6 Infected wounds generally heal through either secondary intention, by which healing occurs naturally without surgical intervention, or tertiary intention (delayed surgical closure), in which suturing is done after reduction in pus and the development of a significant amount of fresh granulation tissue.7-9 Natural healing often requires approximately 21 days, which may be excessively prolonged for pediatric patients who are uncooperative with treatment.8 Additionally, regular dressing changes or secondary suturing can cause pain and anxiety in pediatric patients.8,10 Thus, it is essential to seek a closure therapy for cutaneous abscesses that avoids the use of needle suturing, provides pain-free closure, and is well-suited to pediatric outpatient use.
The novel zipper device (Shandong Shirui Co, Ltd), typically consisting of 2 adhesive-based strips with attached ratchet straps, is a noninvasive, sutureless, and user-friendly method for wound closure of surgical incisions, providing dynamic traction force and a protective zone.11,12 When applied to both sides of the lesion, the zipper device effectively closes wounds using interconnected nylon straps resembling zip ties, with each pair linked by a longitudinal nylon strap to ensure even traction force distribution on the adhesive strips between them. The device’s design also allows for adjustable closure; for example, the ratchet straps can be loosened if excessive tension is applied during closure.11,13,14 According to Ma et al,15 the use of a zipper device in an adult wound care center significantly reduced the healing time for infected incisions by approximately 12 days compared with secondary suturing, without hindering drainage. However, Ma et al15 did not report the relationship between the timing of zipper interventions and wound healing time, leading to variations in treatment outcomes due to differing protocols, which may introduce bias in research findings.
The current pilot case series evaluates the feasibility and effectiveness of a novel zipper device in achieving pain relief and reduced wound healing time in pediatric simple abscesses. It also further analyzes the dose-response relationship between the intervention interval (ie, time from I&D to zipper application) and total healing time, providing insights into optimizing pediatric cutaneous abscess treatment with the zipper device
Materials and Methods
Participant enrollment
The research was approved by the Human Research Ethics Committee of the Children’s Hospital of Chongqing Medical University (CHCMU), Chongqing, China. Written informed consent was obtained from the legal guardians of all the pediatric patients involved in the study. The study focused on simple cutaneous abscesses, excluding multiple abscesses, cellulitis, epidermal skin infections, small abscesses (<1 cm), and systemic infections (characterized by fever, chills, or hypotension). Patients who required general anesthesia-assisted I&D, lacked purulent discharge, and had immunodeficiency disorders (including steroid use and coagulation abnormalities) and allergic dermatological conditions were also excluded.
Therapy methods
To determine the extent of infection, physical examination and ultrasound were performed. While physical examination identifies skin abscesses through pain, erythema, warmth, edema, and induration, with palpable fluctuance indicating purulent collection, ultrasound assesses infection depth and its relationship with surrounding tissues.5 The size of the infected area was evaluated by measuring the extent of erythema and induration. The length and width of the infected region were measured, with the length aligned with the body’s longitudinal axis and the width assessed perpendicular to this axis.
From September 2023 through September 2024, 26 pediatric patients with uncomplicated cutaneous abscesses were treated at CHCMU’s outpatient department using zipper application after I&D. The procedure of I&D was performed under local anesthesia along dermatoglyphic lines, followed by irrigation of the abscess cavity with hydrogen peroxide, 0.5% povidone-iodine solution, and saline. The abscess cavity was then packed with calcium alginate dressing and topical antibiotic ointment, and the wound was subsequently covered with sterile gauze. Placement of the novel zipper device (Shandong Shirui Co, Ltd) on the wound margins occurred after draining decreased and fresh granulation tissue emerged (Figure 1).
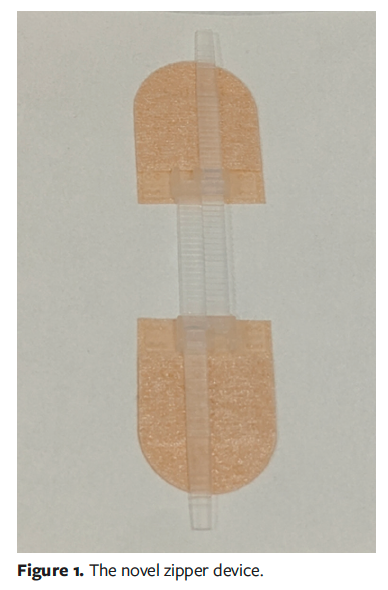
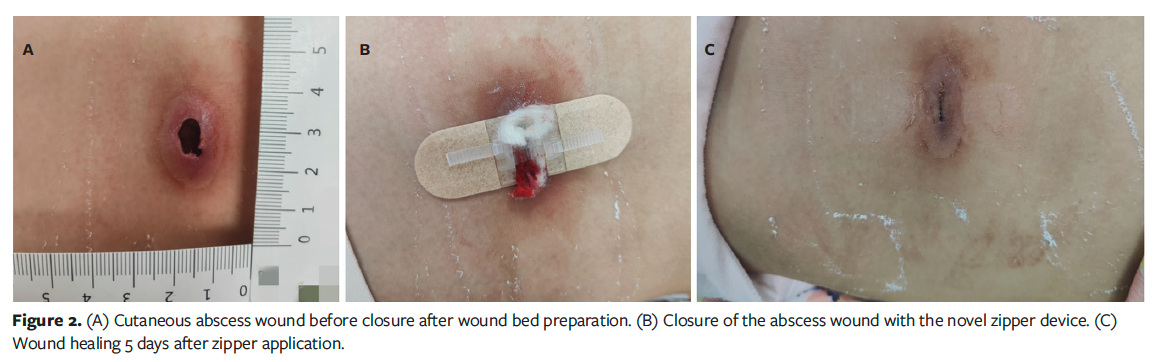
Initially, a minor traction force was applied to the zipper device, maintaining a gap width between the wound edges for drainage and promoting endothelial cell migration.13 This gap enables dressing replacement and wound monitoring without removing the zipper, when the zipper remains intact and does not need substitution.15 In the current case series, as drainage diminished and a significant amount of granulation tissue developed, the traction force was gradually increased to align the skin margins until closure. The zipper remained in situ until complete wound healing. Prior to zipper application, wound dressings were inspected and changed daily; however, the frequency was adjusted to every 2 days or 3 days after zipper placement, with corresponding adjustments to the traction force (Figure 2).
Measurement indicators
Healing time was defined as the duration from I&D of the cutaneous abscess to complete wound closure. During application and removal of the zipper, pain was assessed using the Face, Legs, Activity, Cry, and Consolability (FLACC) observation scale, which has been validated for accurate pain evaluation in pediatric patients aged up to 19 years.16,17 The FLACC scale assesses pediatric pain through 5 behavioral domains: Face, Legs, Activity, Cry, and Consolability. Each category is scored 0-2, yielding a total score of 0-10 (1-3: mild pain, 4-6: moderate pain, 7-10: moderate to severe pain). Higher scores indicate greater pain intensity and discomfort. As noted above, the intervention interval spans from the time of I&D of the abscess to the first application of the novel zipper.
Statistical analysis
Demographic statistics and clinical outcomes were collected using a case record form, and wound care was conducted by a dedicated wound specialist nurse (X.M.). Pain score was assessed by the other wound specialist nurse (X.Y.). A follow-up telephone call (M.W.) was performed on day 7 post-healing to assess the potential for wound dehiscence and abscess recurrence. The data were analyzed with SPSS version 27.0 (IBM Corporation) and R software (version 4.4.1; The R Project for Statistical Computing). Continuous variables are expressed as mean (SD), and categorical variables are expressed as frequency (%). Linear regression determined factors linked to wound healing time (dependent variable) and was verified by the Durbin-Watson statistic, with alpha set at .05 as the level of significance. The restricted cubic spline (RCS) model was used to analyze the association between the intervention interval using the novel zipper post-I&D and healing time.
Results
Demographic baseline of participants
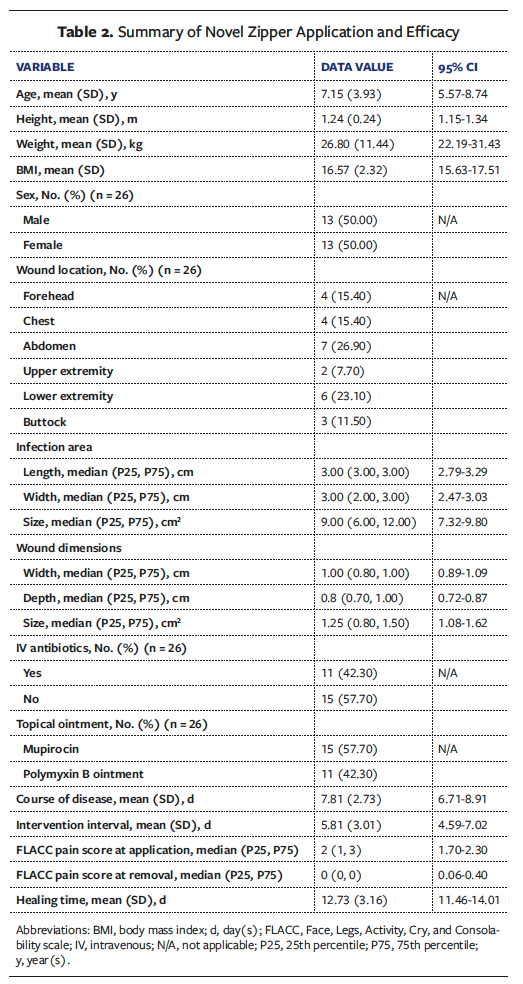
The mean (SD) patient age was 7.15 (3.93) years (95% CI, 5.57-8.74), with a diverse age range from young children to adolescents. Body mass index (BMI) averaged 16.57 (2.32) (95% CI, 15.63-17.51), indicating a healthy weight status. The mean course of disease was 7.81 ± 2.73 days (95% CI, 6.71-8.91). Abscess distribution among the 26 patients included the forehead (n = 4), chest (n = 4), abdomen (n = 7), upper limbs (n = 2), lower limbs (n = 6), and buttocks (n = 3), with the abdomen (26.9%) and the lower extremities (23.1%) predominating. Median infection area measured 9 cm2 (95% CI, 7.32-9.80). Median wound size after I&D was 1.25 cm2 (95% CI, 1.08-1.62) (Tables 1 and 2).
Measurement outcomes
The mean (SD) intervention interval was 5.8 (3.01) days (95% CI, 4.59-7.02). The healing time averaged 12.73 (3.16) days (95% CI, 11.46-14.01). The median FLACC pain score was 2 during zipper placement, decreasing to 0 after the completion of therapy, indicating a significant reduction in pain (Tables 2 and 3). Notably, all participants completed treatment, with no wound complications reported.
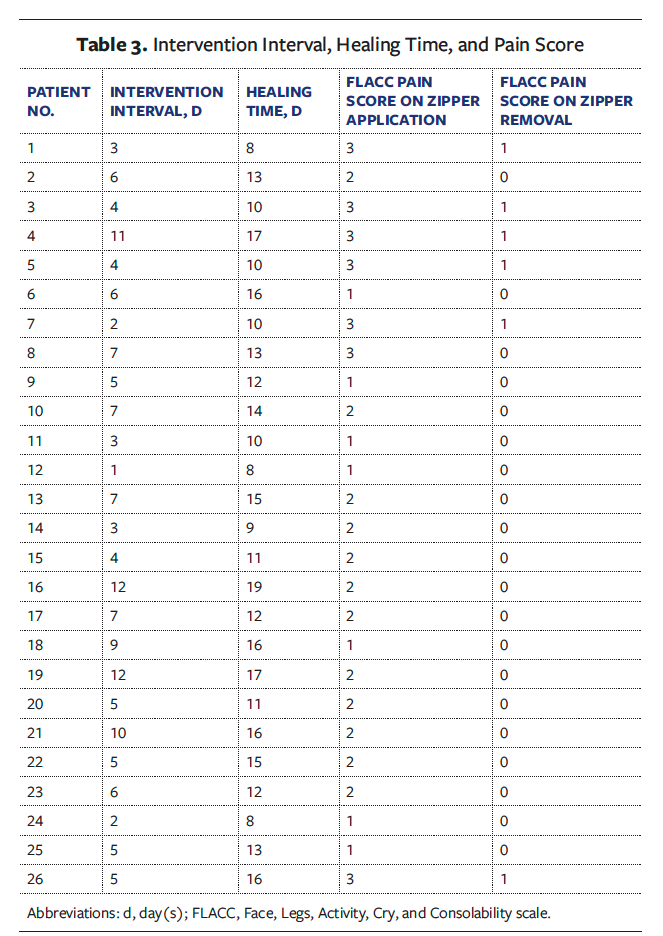
Linear regression analysis
The simple linear regression model showed no significant association between wound healing time and age, sex, BMI, course of disease, wound size, or intravenous antibiotic use (Table 4). However, there was a correlation between cutaneous abscess dimensions (length, width, size) (P < .05) and the intervention interval (B = 0.92, SE = 0.10, β = 0.88, t = 9.044, P < .001) with wound healing time. A multiple linear regression analysis was conducted using 2 models: Model 1 represented the relationship among infection metrics, the intervention interval, and the wound healing time, and model 2 represented the stepwise multicollinearity analysis. The analysis revealed that infection size (B = 0.260, SE = 0.090, β = 0.260, t = 2.924, P = .008) and the intervention interval (B = 0.850, SE = 0.090, β = 0.810, t = 9.187, P = .001) were both predictive of wound healing time (Table 5). The model explained 83.5% of variance, supported by a Durbin-Watson statistic of 2.122 (in proximity to the optimal value of 2.0), and the standardized residuals were normally distributed.
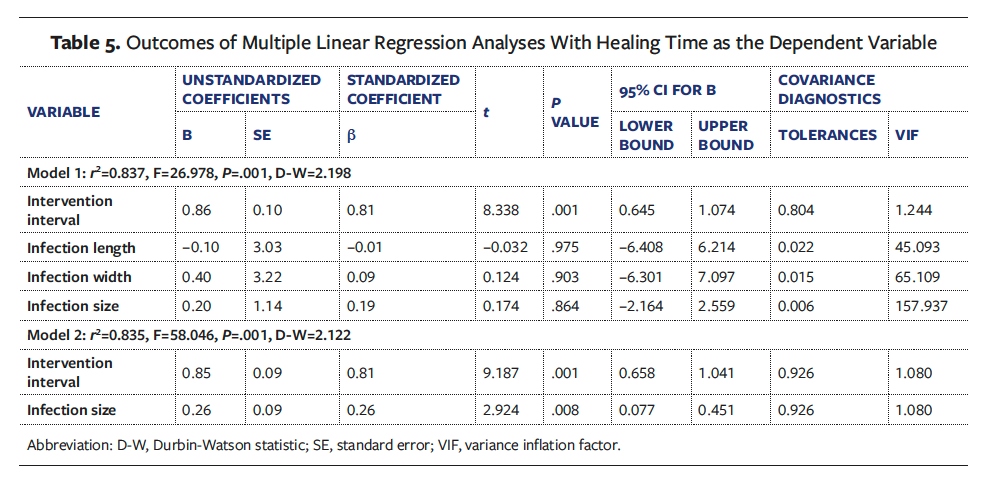
RCS analysis
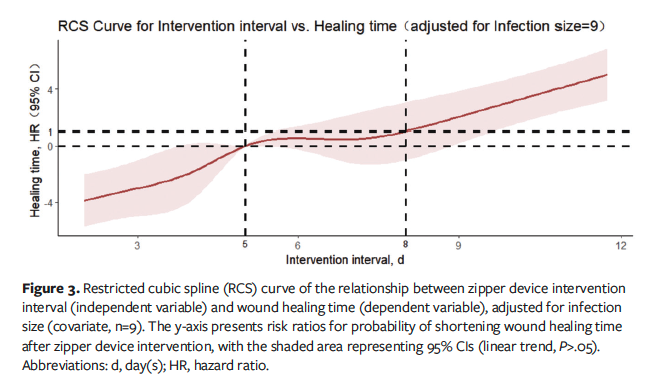
Adjusting for the infection size and selecting 5 nodes for fitting, the RCS analysis in model 2 revealed a J-shaped relationship between the intervention interval and healing time (Ptotal < .001, PNonlinear = .406), indicating that wound healing time decreased with a shorter intervention interval of zipper placement until approximately 5 days, after which wound healing time plateaued and then increased rapidly beyond the eighth day (Figure 3). In addition, the infection size showed no significant effect on wound healing time (P = .152).
Discussion
The current case series involved 26 pediatric patients, predominantly school-aged children with an average age of 7.1 (3.93) years. In contrast, Chumpitazi et al4 investigated a cohort primarily consisting of preschoolers averaging 3.1 (IQR 1.7) years. This age discrepancy suggests that children from preschool to school age are particularly susceptible to S aureus, as this age group is more likely to experience nasal colonization by S aureus, as reported by Zhang et al.3 Additionally, in the current case series, the use of intravenous antibiotics demonstrated no significant influence on wound healing time following I&D of skin abscesses, consistent with Lee et al18 and López et al,8 suggesting that intravenous antibiotics do not affect the outcome for uncomplicated pediatric skin abscess management post-I&D.
In the current series, the mean healing time with zipper implementation was 12.73 (3.16) days, which is respectively 25% and 40% faster than that of secondary suturing (15 [7.5] days)7 and natural healing (typically 21 days).8 These outcomes are in accordance with those of Ma et al,15 who applied zippers to incision infections after hepatobiliary surgery and showed that the healing time with zippers was superior to that with secondary sutures. This result may relate to the tension induced by the novel zipper on the wound. The novel zipper provides bidirectional traction along the wound margins using ratchet straps, minimizing lateral stress concentrations to maintain a low-tension healing environment within the wound. The zipper also establishes a protective barrier to reduce external friction and injuries, providing a stable healing environment.13,19,20 Studies show that during the wound healing process, a low-tension environment can effectively stimulate the proliferation and migration of fibroblasts and epithelial cells, enhancing the development of collagen and extracellular matrix, and also improving blood circulation and metabolic exchange to accelerate healing.21,22
Pain is inevitable in infected wound treatment, particularly in pediatric cases in which frequent dressing changes lead to pain, fear, and posttraumatic stress disorder.10,23 The current study found that pain caused by the zipper was mild during placement (median score of 2) and diminished during removal, which is significantly lower than previously reported natural healing (mean [SD] 9.36 [0.65])24 and suturing (5.1 [0.8])25 scores. Although the current case series did not directly observe the following finding, Shrestha et al11 revealed that the zipper method reduced incision closure time by approximately 5 minutes compared with suturing. The time savings with and simplicity of the zipper device make it a practical noninvasive alternative for treatment of pediatric skin abscesses, notably reducing treatment-related trauma in outpatient settings.
Closure management of the infected wound depends on healthy granulation tissue formation, which is marked by necrotic tissue removal and resolved inflammation following I&D or debridement.26 Singh et al27 and Ma et al15 suggested that it was safe to close infected incisions when drainage fluid significantly decreased and pink granulation tissue appeared. Fresh granulation tissue with a reddish granular appearance containing capillaries and essential extracellular matrix components develops during the proliferative phase (from day 4 to day 20 postinjury), progressively filling the wound bed to facilitate reepithelialization and wound closure.28,29 However, abscess environments hinder this process due to the presence of pus and necrotic tissues. Meanwhile, the biofilms formed by S aureus negatively affect antibiotic effectiveness, induce chronic inflammation, and prolong the proliferative healing phase, resulting in disruption of wound healing balance and delaying the overall process.3,28,30,31
Definitive evidence on optimal closure timing for infected wounds is lacking, and assessments of granulation tissue maturity vary among wound specialist nurses, contributing to inconsistent treatment outcomes. The current case series determined the best timing for zipper use to establish evidence-based parameters by analyzing the dose-response relationship between the intervention interval and healing time. Linear regression suggested that both infection size and intervention interval significantly affected wound healing time, with larger infection areas extending healing times, aligning with the findings of Chumpitazi et al,4 and that longer intervals before zipper application prolonged healing. The RCS curve analysis showed a J-shaped linear correlation between the intervention interval and healing time (Ptotal < .001, Pnonlinear = .406), which suggests the optimal timing of zipper use is 3 days to 5 days post-I&D to significantly shorten healing time. However, beyond this period, healing time plateaued (5 days-8 days) and subsequently increased (>8 days), indicating that late placement does not reduce wound healing time. This suggestion aligns with the physiological wound healing process, in which granulation tissue begins to form around the fourth day postinjury.28 Use of the zipper during this period can reduce the wound tension by its bidirectional traction mechanism, thereby promoting the growth of granulation tissue and thus accelerating wound healing.21,22 Singh et al27 also indicated delayed primary closure on the fifth day postoperatively to be safe for healing infected incisions.
Limitations
While this case series provides quantitative evidence for the optimal intervention timing of novel zipper device use in pediatric cutaneous abscesses, it has certain limitations. The major drawback is that it is a small-scale case series conducted at a single center and including a single disease type. Additionally, it lacks control groups with alternative closure methods, such as suturing or sterile strips, which restricted direct efficacy comparisons. Future investigations should involve larger enrollment in multicenter randomized controlled trials across wound types in pediatric populations.
Conclusion
This case series confirms the novel zipper device as a practical noninvasive alternative approach for pediatric cutaneous abscess wound closure in outpatient settings, demonstrating its effectiveness in reducing healing time and alleviating pain. The RCS analysis indicates the fifth day post-I&D is a critical juncture and highlights that the optimal period for zipper device use to shorten wound healing is 3 days to 5 days following I&D of the skin abscess. A future prospective comparative study examining the efficacy of zippers, sterile strips, and sutures in the closure of abscessed wounds in pediatric patients is needed to provide essential evidence to support the optimization of outpatient wound care protocols for pediatric cutaneous abscesses.
Author and Public Information
Authors: Wei Bingting, MS1,2,3; Yang Chen, MS, RN1,2,3; Ma Wei, MS1,2,3; Xie Mingjing, BS,RN1,2,3; Xiong Yang, BS, RN1,2,3; Wu Tingting, BS, RN1,2,3; and Zhang Xuebing, MD, RN1,2,3
Acknowledgments: 1Department of Day Surgery, Children’s Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorder, Chongqing, China; 2China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Chongqing, China; 3Chongqing Key Laboratory of Structural Birth Defect and Reconstruction, Chongqing, China
Author Contributions: W.B. and Y.C. both equally contributed to the conception, design, data analysis, interpretation, and manuscript drafting/revision. M.W., X.M., X.Y., and W.T. collected human material and edited the manuscript. Z.X. and M.W. critically revised and approved the final version.
Funding: Funding for this work came from the fourth batch of hospital-level scientific research projects of nursing discipline in Children's Hospital Affiliated to Chongqing Medical University (CHCQMU2023.15).
Disclosure: The authors disclose no other financial or other conflicts of interest.
Ethical Approval: Approval was obtained from the Institutional Review Board of Children’s Hospital of Chongqing Medical University (No.2023342). Written informed consent was obtained from all participants included in the study.
Correspondence: Zhang Xuebing, Department of Day Surgery, Children’s Hospital of Chongqing Medical University, No.20, Jinyu Avenue, Kangmei Street, Yubei District, Chongqing, 400014, China; zh651221200@163.com
Manuscript Accepted: June 24, 2025
References
1. Galli L, Venturini E, Bassi A, et al. Common community-acquired bacterial skin and soft-tissue infections in children: an intersociety consensus on impetigo, abscess, and cellulitis treatment. Clin Ther. 2019;41(3):532-551.e517. doi:10.1016/j.clinthera.2019.01.010
2. Sami Awayid H, Qassim Mohammad S. Prevalence and antibiotic resistance pattern of methicillin-resistant Staphylococcus aureus isolated from Iraqi hospitals. Arch Razi Inst. 2022;77(3):1147-1156. doi:10.22092/ARI.2022.357391.2031
3. Zhang C, Liang B, Xiong Z, et al. Distribution of biocide resistance genes and association with clonal complex genotypes in Staphylococcus aureus isolated from school-age children in Guangzhou. Infect Drug Resist. 2022;15:7165-7175. doi:10.2147/IDR.S387528
4. Chumpitazi CE, Rees CA, Camp EA, et al. Factors influencing drainage setting and cost for cutaneous abscesses among pediatric patients. Am J Emerg Med. 2017;35(2):326-328. doi:10.1016/j.ajem.2016.10.031
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52. doi:10.1093/cid/ciu444
6. Menegas S, Moayedi S, Torres M. Abscess management: an evidence-based review for emergency medicine clinicians. J Emerg Med. 2021;60(3):310-320. doi:10.1016/j.jemermed.2020.10.043
7. Singer AJ, Taira BR, Chale S, Bhat R, Kennedy D, Schmitz G. Primary versus secondary closure of cutaneous abscesses in the emergency department: a randomized controlled trial. Acad Emerg Med. 2013;20(1):27-32. doi:10.1111/acem.12053
8. López J, Gómez G, Rodriguez K, Dávila J, Núñez J, Anaya L. Comparative study of drainage and antibiotics versus drainage only in the management of primary subcutaneous abscesses. Surg Infect (Larchmt). 2018;19(3):345-351. doi:10.1089/sur.2017.225
9. Almadani YH, Vorstenbosch J, Davison PG, Murphy AM. Wound healing: a comprehensive review. Semin Plast Surg. 2021;35(3):141-144. doi:10.1055/s-0041-1731791
10. Walburn J, Vedhara K, Hankins M, Rixon L, Weinman J. Psychological stress and wound healing in humans: a systematic review and meta-analysis. J Psychosom Res. 2009;67(3):253-271. doi:10.1016/j.jpsychores.2009.04.002
11. Shrestha O, Basukala S, Karki S, et al. Comparison between novel zipper device and conventional methods for skin closure: a systematic review and meta-analysis. Ann Med Surg (Lond). 2024;86(3):1631-1640. doi:10.1097/MS9.0000000000001769
12. Chen YH, Chai MY, Xuan CK, et al. Tuning the properties of surgical polymeric materials for improved soft-tissue wound closure and healing. Progress in Materials Science. 2024;143. doi:10.1016/j.pmatsci.2024.101249
13. Levi K, Ichiryu K, Kefel P, et al. Mechanics of wound closure: emerging tape-based wound closure technology vs. traditional methods. Cureus. 2016;8(10):e827. doi:10.7759/cureus.827
14. Menkowitz B, Olivieri G, Belson O. Patient satisfaction and cosmetic outcome in a randomized, prospective study of total knee arthroplasty skin closure comparing zip surgical skin closure with staples. Cureus. 2020;12(1):e6705. doi:10.7759/cureus.6705.
15. Ma WJ, Zhou Y, Mao H, et al. Healing time of incision infection after hepatobiliary surgery treated by needle-free incision suture closure. World J Gastroenterol. 2014;20(42):15815-15819. doi:10.3748/wjg.v20.i42.15815
16. Malviya S, Voepel-Lewis T, Burke C, Merkel S, Tait AR. The revised FLACC observational pain tool: improved reliability and validity for pain assessment in children with cognitive impairment. Paediatr Anaesth. 2006;16(3):258-265. doi:10.1111/j.1460-9592.2005.01773.x
17. Crellin DJ, Harrison D, Santamaria N, Huque H, Babl FE. The psychometric properties of the FLACC scale used to assess procedural pain. J Pain. 2018;19(8):862-872. doi:10.1016/j.jpain.2018.02.013
18. Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23(2):123-127. doi:10.1097/01.inf.0000109288.06912.21
19. Aragona M, Sifrim A, Malfait M, et al. Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature. 2020;584(7820):268-273. doi:10.1038/s41586-020-2555-7
20. Kulkarni AG, Tapashetti S, Patel PM. Use of zipper ratcheting straps as a bailout for postoperative cerebrospinal fluid leaks: our experience. Global Spine J. 2020;10(4):443-447. doi:10.1177/2192568219860463
21. Harn HIC, Ogawa R, Hsu CK, Hughes MW, Tang MJ, Chuong CM. The tension biology of wound healing. Exp Dermatol. 2019;28(4):464-471. doi:10.1111/exd.13460
22. Berry CE, Downer Jr M, Morgan AG, et al. The effects of mechanical force on fibroblast behavior in cutaneous injury. Front Surg. 2023;10:1167067. doi:10.3389/fsurg.2023.1167067
23. Gardner SE, Abbott LI, Fiala CA, Rakel BA. Factors associated with high pain intensity during wound care procedures: a model. Wound Repair Regen. 2017;25(4):558-563. doi:10.1111/wrr.12553
24. Yang C, Wang S, Li CC, et al. A high-vacuum wound drainage system reduces pain and length of treatment for pediatric soft tissue abscesses. Eur J Pediatr. 2017;176(2):261-267. doi:10.1007/s00431-016-2835-2
25. Ni Z, Sun J, Qi S. Therapeutic effect of topical negative pressure therapy/vacuum-associated closure therapy on cephalic facial skin abscess. Surg Infect (Larchmt). 2020;21(8):722-725. doi:10.1089/sur.2019.184
26. Eliya-Masamba MC, Banda GW. Primary closure versus delayed closure for non bite traumatic wounds within 24 hours post injury. Cochrane Database Syst Rev. 2013;2013(10):CD008574. doi:10.1002/14651858.CD008574.pub3
27. Singh PK, Sethi MK, Mishra TS, et al. Comparison of surgical site infection (SSI) between negative pressure wound therapy (NPWT) assisted delayed primary closure and conventional delayed primary closure in grossly contaminated emergency abdominal surgeries: a randomized controlled trial. Langenbecks Arch Surg. 2023;409(1):19. doi:10.1007/s00423-023-03202-x
28. Tapiwa Chamanga E. Clinical management of non-healing wounds. Nurs Stand. 2018;32(29):48-63. doi:10.7748/ns.2018.e10829
29. Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. doi:10.1098/rsob.200223
30. Niculescu AG, Georgescu M, Marinas IC, et al. Therapeutic management of malignant wounds: an update. Curr Treat Options Oncol. 2024;25(1):97-126. doi:10.1007/s11864-023-01172-2
31. Atkin L. Chronic wounds: the challenges of appropriate management. Br J Community Nurs. 2019;24(Sup9):S26-S32. doi:10.12968/bjcn.2019.24.Sup9.S26


















