Transforming Powder Dressing for Lower Extremity Wounds in Patients with Diabetes: A Multinational Case Series
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. Chronic lower extremity wounds in patients with diabetes are a significant clinical and economic burden. Traditional dressings have poor healing rates and require frequent changes, burdening patients and caregivers. Objective. To evaluate if transforming powder dressing (TPD), a hydrophilic polymer powder that forms a moisture-retentive gel conforming to the wound surface, can address these limitations, as clinical data remain limited. Materials and Methods. This retrospective, multinational case series evaluated TPD in 17 patients with diabetic lower extremity wounds across Egypt, India, the United Arab Emirates, and the Palestinian territories. Wound types included diabetic foot ulcers (DFUs), venous ulcers, and postamputation wounds. For all patients, previous standard of care efforts had failed. Results. TPD was associated with consistent wound size reduction (mean [standard deviation (SD)] 80% [10%]), granulation tissue formation, and pain relief. DFUs and non-DFUs showed comparable outcomes, with no significant differences in healing time (7.1 weeks and 5.9 weeks, respectively; P = .39). Dressings were changed every 5 to 10 days, and no complications were reported. Conclusion. TPD appears to be a safe, well-tolerated, and effective adjunct for managing diabetic lower extremity wounds. Its ability to promote healing while reducing pain and dressing frequency offers clinical and logistical benefits. Larger, prospective studies are needed to validate these findings and guide treatment protocols.
Introduction
Chronic lower extremity wounds represent a persistent and growing clinical challenge, particularly in patients with diabetes mellitus (DM). Sequelae of diabetes—such as peripheral neuropathy (PN), microvascular disease, immune dysfunction, and chronic inflammation—result in loss of sensation, unnoticed injuries, and impaired healing.1,2 Diabetic foot ulcers (DFUs) commonly develop on pressure-bearing areas, often following unrecognized repetitive trauma in the setting of PN.3 Other chronic wounds seen in patients with diabetes, such as venous stasis ulcers and arterial insufficiency ulcers, may arise in the absence of a specific traumatic insult and instead are driven by underlying vascular pathology.3
Estimates are that up to 34% of persons with diabetes are expected to develop a DFU during their lifetime.4 These wounds are a major cause of infection and hospitalization, and they contribute significantly to health care costs and a diminished quality of life.5 Traditional wound care approaches, such as saline-soaked gauze, hydrocolloid dressings, and topical antimicrobials, aim to maintain a moist environment that is conducive to healing. However, the frequent dressing changes required can disrupt fragile granulation tissue and exacerbate patient discomfort, leading to poor adherence on the part of the patient. This can further increase the risk of infection and amputation.6-8 Approximately 20% of patients with a DFU who require lower extremity amputation will die within 1 year of surgery.9,10
Advanced wound dressings have emerged as promising treatment alternatives. One such innovation is transforming powder dressing (TPD), a powder composed of hydrophilic polymer particles that forms a moist, semiocclusive matrix when exposed to wound exudate.11-13 The powder form allows easy conformation of the product to wounds with irregular contours or deep tissue involvement—common features of diabetic lower extremity wounds.13
Although the theoretical advantages of TPD are well established, clinical evidence evaluating its effectiveness—particularly in the context of diabetic lower extremity wounds—remains limited. Existing reports are primarily anecdotal or based on single-center case reports, many of which lack robust outcome assessments or contextualization within broader wound care literature.11,14
This retrospective, multinational case series sought to address these gaps by evaluating the real-world use of TPD in managing diabetic lower extremity wounds. The study included 17 cases from 4 regions and analyzes key outcomes such as healing time, frequency of dressing changes, pain reduction, and complications. By presenting outcomes in a diverse patient population across a range of clinical settings, this study aimed to provide actionable insights into the clinical utility of TPD and its potential role in optimizing care for chronic wounds in patients with diabetes.
Materials and Methods
This retrospective, multicenter case series evaluated the clinical performance of TPD in 17 patients with diabetic lower extremity wounds, treated across 4 regions—Egypt, India, the United Arab Emirates, and the Palestinian territories—between January 2019 and December 2020.
Eligible patients had a documented diagnosis of DM and a lower extremity wound of at least 1 week’s duration that had not improved with standard of care (SOC). Included wounds varied in etiology and location, encompassing DFUs, postamputation wounds, and venous stasis ulcers. Patients with nondiabetic wounds, systemic infection or sepsis, or incomplete treatment records were excluded.
Before TPD was initiated, patients had received SOC treatments, including saline-moistened gauze, antiseptic dressings, and off-loading. The protocol for TPD application included moistening the wound with normal saline, applying TPD uniformly to the wound, and covering it with a secondary dressing—including gauze, paraffin gauze, foam padding, or Microdacyn solution (hereafter “superoxidized hypochlorous acid and hypochlorite solution”; Te Arai BioFarma Ltd)—which was selected based on wound characteristics and institutional protocols. Dressings were typically changed every 5 to 10 days.
Clinical and demographic data were extracted from patient records, including age, sex, wound type and location, wound duration prior to TPD use, frequency of dressing changes before and during TPD treatment, total number of dressing changes, complications, and degree of wound healing. DFUs were classified by Wagner grade, and non-DFUs were described using clinical features. The primary outcome was time to complete or near-complete epithelialization. Secondary outcomes included number and interval of dressing changes, pain reduction, granulation tissue formation, percentage wound size reduction, and complications.
Descriptive statistics were calculated for continuous and categorical variables. Group comparisons between DFUs and non-DFUs were performed using t tests and chi-square or Fisher exact tests, with P values less than .05 considered statistically significant. Pearson correlation was used to assess whether wound duration prior to TPD correlated with time to healing. Analyses were conducted using R software (version 4.3.2; The R Project for Statistical Computing).
This study complied with the Declaration of Helsinki. Institutional review board approval was not required due to the study’s retrospective design and the use of de-identified data.
Results
Table 1 summarizes patient and wound characteristics by group. Among the 17 cases, the mean (SD) age was 57.4 (7.6) years, and most patients were male (76.5% [n = 13]); there were 4 female patients (23.5%). All patients had DM, and common comorbidities included neuropathy, cardiovascular disease (CVD), and peripheral arterial disease. Three patients (17.6%) had a history of lower limb amputation.
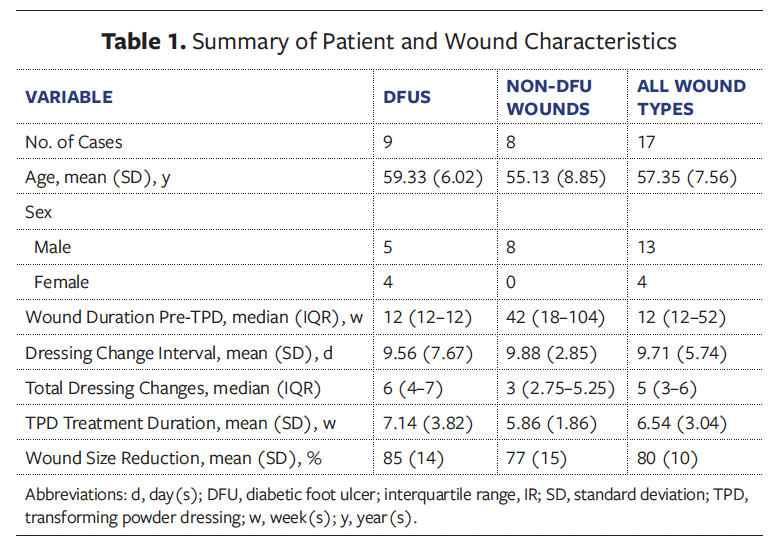
The median (interquartile range [IQR]) wound duration before TPD treatment was 12 weeks (12 weeks–52 weeks), and the mean (SD) dressing change interval was 9.7 (5.7) days. Patients underwent a median (IQR) of 5 (3–6) total dressing changes, with a mean (SD) TPD treatment duration of 6.5 (3.0) weeks and a mean wound size reduction of 80% (10%). No complications were reported across the cohort.
When stratified by wound type, mean patient age was higher in the 9 patients with DFUs (59.3 [6.0] years) than in the 8 patients with non-DFU wounds (55.1 [8.9] years); additionally, patients with DFUs had a shorter median wound duration prior to TPD treatment compared with patients with non-DFU wounds (12 weeks vs. 42 weeks). DFUs required more total dressing changes than non-DFUs (median of 6 and 3, respectively) and had a longer average healing time, with a mean (SD) treatment duration of 7.1 (3.8) weeks vs. 5.9 (1.9) weeks (P = .39). Mean wound size reduction was greater in DFUs (85% [14%]) than in non-DFUs (77% [15%]); this difference was not statistically significant (P = .27).
Pearson correlation analysis revealed no significant relationship between wound chronicity and time to healing (r = 0.056, P = .83), suggesting that healing outcomes were not dependent on wound duration prior to TPD initiation.
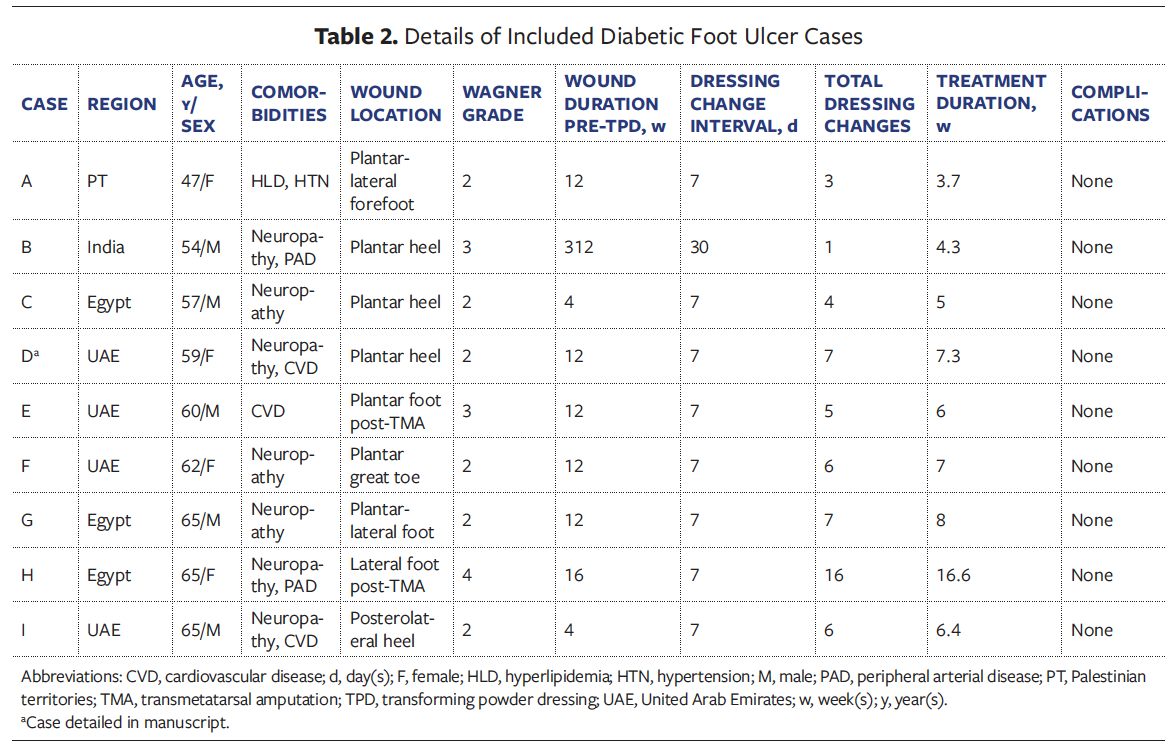
Most wounds were located on the foot (94.1% [n = 16]); 1 was located on a post-
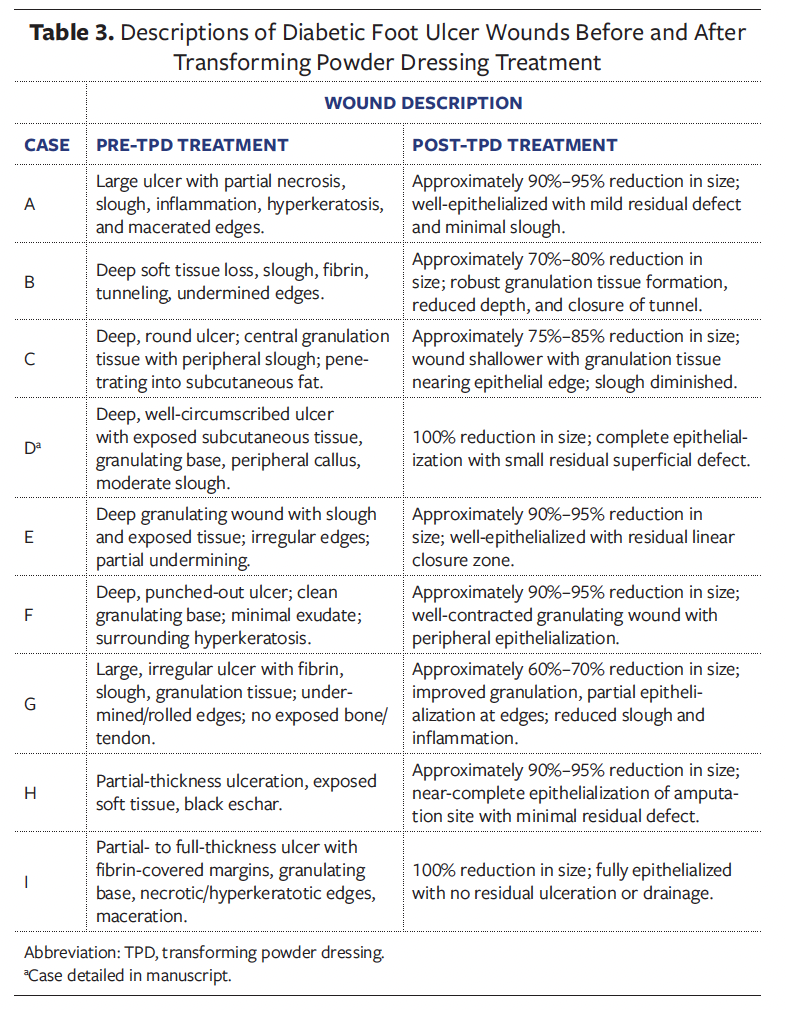
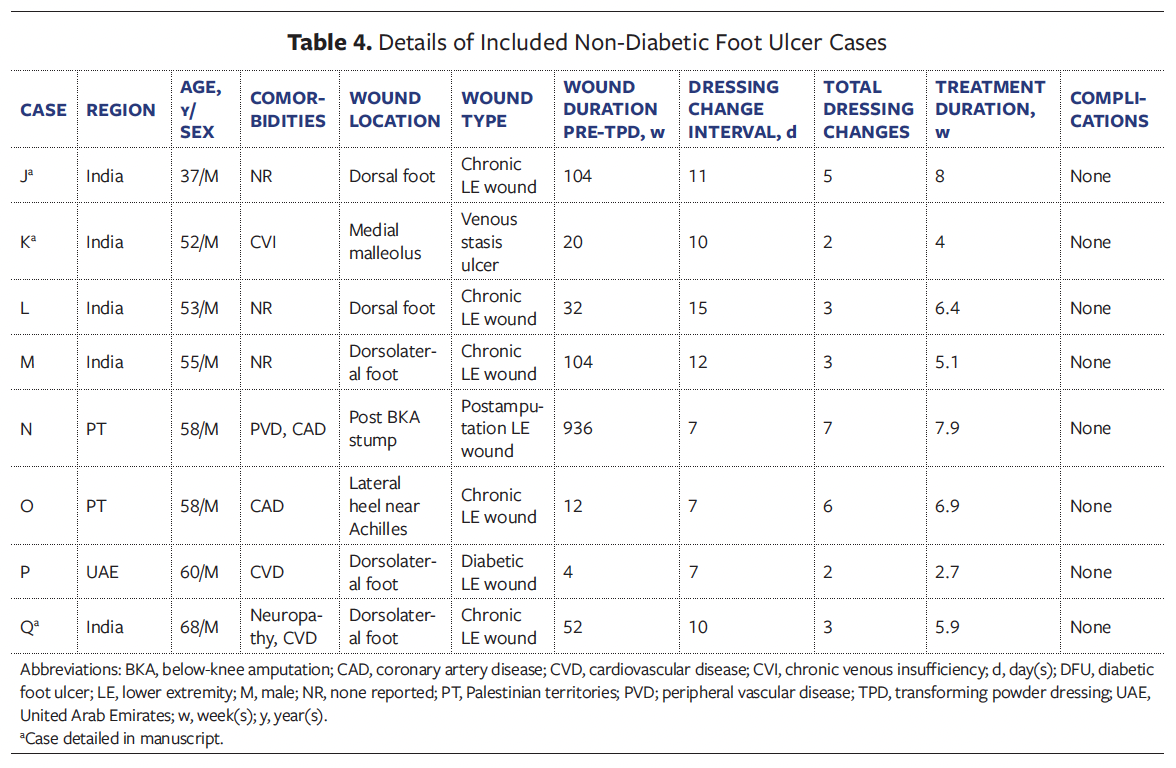
amputation residual limb. Of the DFUs, 6 were Wagner grade 2, 2 were grade 3, and 1 was grade 4 (Tables 2, 3). Non-DFU wounds included venous ulcers, postoperative wounds, and atypical diabetic lower extremity wounds (Table 4, 5).
For all patients, previous standard wound care regimens had been unsuccessful. For the TPD treatment presented in this study, secondary dressings—including gauze, foam, paraffin gauze, and superoxidized hypochlorous acid and hypochlorite—were selected based on wound characteristics and institutional protocols.
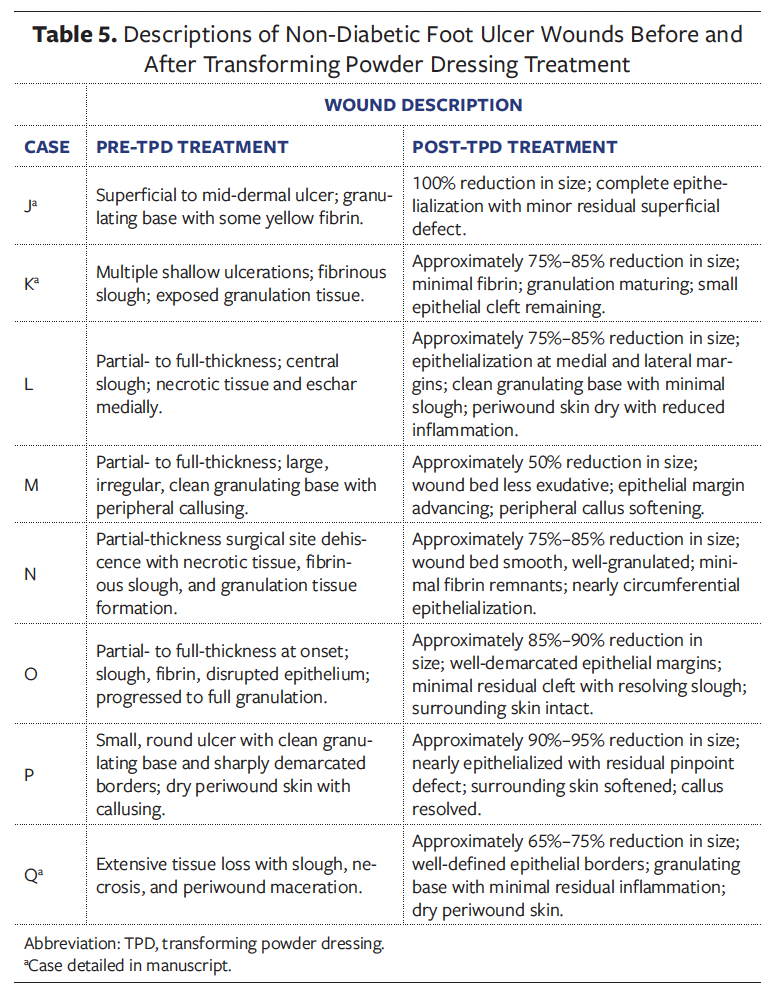
Case D
A 59-year-old female in the UAE with DM, CVD, and PN presented with a chronic DFU on the plantar heel that had been present for 3 months. The ulcer was Wagner grade 2, characterized by a deep, well-circumscribed wound with exposed subcutaneous tissue, a granulating base, peripheral callus, and moderate slough (Figure 1). It had previously been managed with antiseptic gauze dressings without improvement. TPD was introduced as the primary dressing; it was applied to the wound bed and then covered by foam padding and sterile gauze. Dressing changes occurred every 7 days, with a total of 7 changes over 51 days. Granulation tissue developed early and progressed steadily. Clinicians noted consistent improvement in wound bed quality. By day 51, the wound had completely epithelialized and required no further dressings. No complications occurred, and surgical intervention was avoided. The patient reported improved comfort and satisfaction with the reduction in dressing changes.
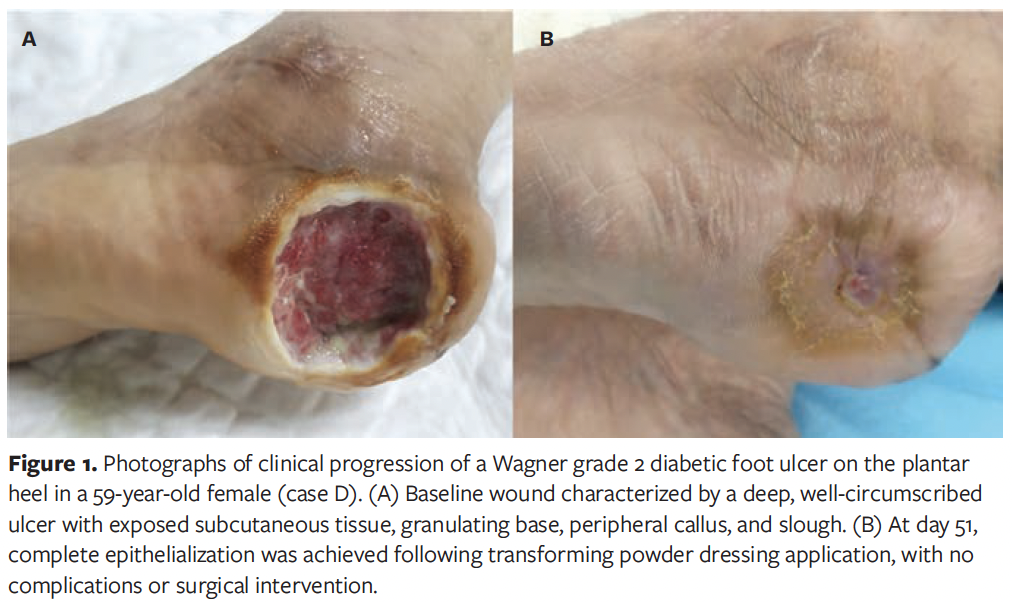
Case J
A 37-year-old male in India with DM presented with a chronic lower extremity wound of 2 years’ duration on the dorsum of the foot. The wound was a superficial to mid-dermal ulcer with a granulating base and areas of fibrin; it was considered at significant risk for surgical escalation (Figure 2). The patient had previously undergone daily dressing changes with antiseptics and gauze, without improvement. TPD was applied directly to the wound bed, followed by superoxidized hypochlorous acid and hypochlorite solution, and sterile gauze. By the second dressing change, visible reduction in wound size and exudate was noted, along with pain improvement. The wound was managed with 5 dressing changes over 56 days, averaging 11 days between changes. Granulation tissue developed consistently, and the wound progressively contracted and epithelialized. By day 56, the wound had completely epithelialized, and amputation was avoided. No complications were reported.
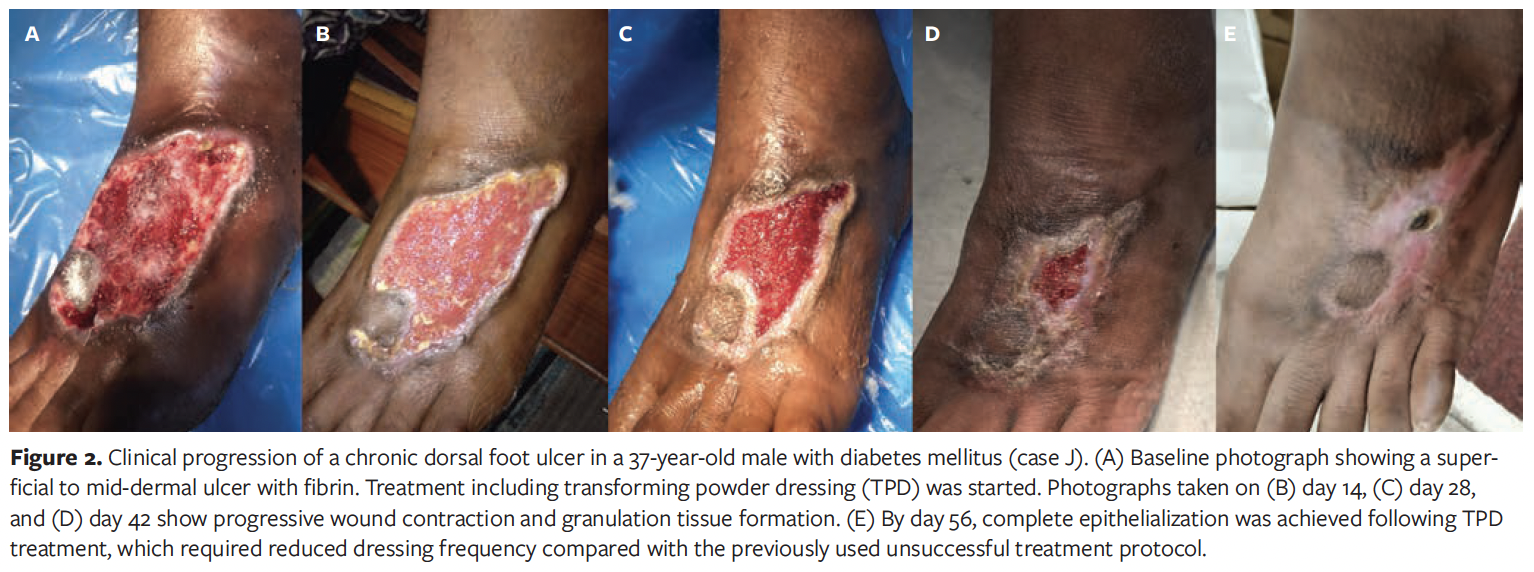
Case K
A 52-year-old male in India with DM and venous insufficiency presented with a venous stasis ulcer at the medial malleolus; the ulcer had been present for 5 months. The wound showed multiple shallow ulcerations with fibrinous slough and exposed granulation tissue (Figure 3). No overt infection was noted, though mild periwound erythema was present. The patient had previously received daily gauze dressings without improvement. TPD was initiated, with superoxidized hypochlorous acid and hypochlorite solution as well as sterile gauze as secondary dressings. No formal debridement was performed. The patient experienced notable pain relief and decreased exudation after TPD initiation. Over 4 weeks, the dressing was changed twice at 10-day intervals. No complications occurred, and epithelialization was nearly complete by day 28. The patient reported improved comfort and satisfaction with the reduction in dressing changes.
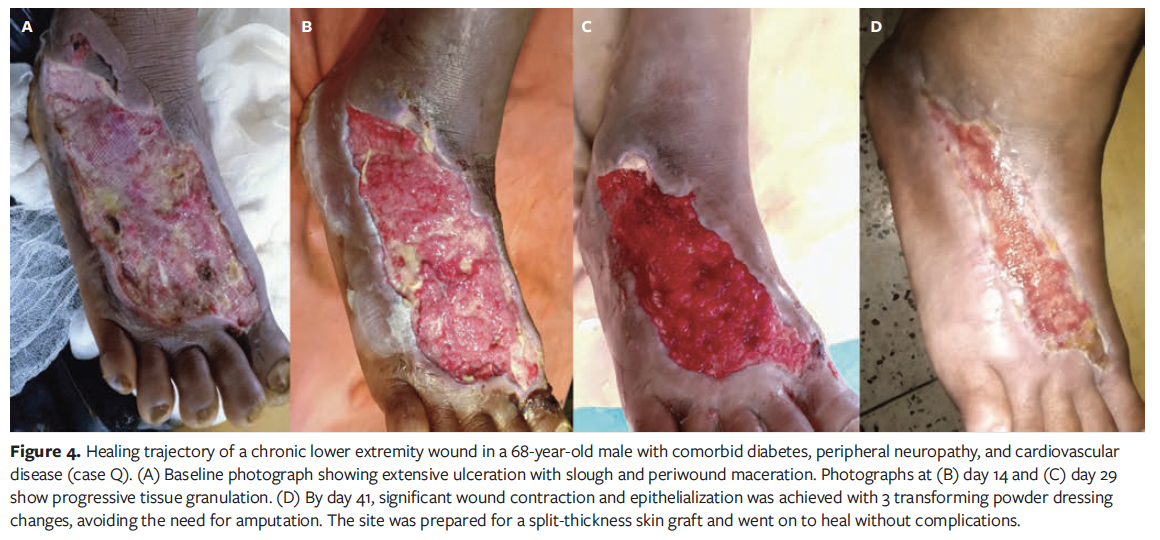
Case Q
A 68-year-old male in India with DM, CVD, and PN presented with a chronic wound of 1 year’s duration on the dorsolateral aspect of the right foot (Figure 4). The ulcer showed extensive tissue loss with slough, necrosis, and periwound maceration. Prior dressings changed every 5 days had failed. Upon starting TPD, the wound was debrided and TPD was applied, with superoxidized hypochlorous acid and hypochlorite solution along with paraffin gauze as secondary dressings. The patient reported notable pain reduction immediately following TPD application. By day 14, wound size had visibly reduced, and by day 29, granulation and contraction progressed. Three dressing changes were performed at 10-day intervals, and by day 41, the wound had reduced in size by 75%, epithelialization was nearly complete, and no complications had occurred. The patient reported improved comfort and smoother dressing changes with TPD. Amputation was avoided, and the site was prepared for a split-thickness skin graft.
Discussion
This multinational case series demonstrates the utility of TPD in treating complex diabetic lower extremity wounds, including DFUs, venous ulcers, and postamputation wounds. Favorable outcomes were observed across diverse wound types and anatomical sites, suggesting that TPD may be a versatile treatment option in real-world diabetic wound care.
TPD’s effectiveness stems from its unique formulation. It contains hydrophilic polymer particles—modified polyacrylates, sodium carboxymethylcellulose, poly-2-hydroxyethyl methacrylate, and poly-2-hydroxypropyl methacrylate—that form a semiocclusive gel upon contact with exudate. Due to its nanoparticulate structure and 7-nm capillary channels, this matrix preserves moisture, promotes autolytic debridement, protects granulation tissue, and conforms to irregular wound beds.13-15 TPD can also be loaded with antiseptics, expanding its antimicrobial utility across various wound types.15
When contextualized against published data, TPD compares favorably to SOC and other advanced dressings. DFU healing rates with SOC are variable; a 2025 meta-
analysis by Coye et al reported a pooled DFU healing rate of only 33.2%.16 In the present series, 7 of 9 DFUs (78%) and 6 of 8 non-DFUs (75%) achieved greater than or equal to 75% epithelialization, with no amputations. These rates exceed those reported for autologous heterogeneous skin constructs—approximately 70% for less severe Wagner grade 1 ulcers—and align with outcomes seen with nanocellulose and hydrogel-based dressings, without their cost or logistical burden.17-19
The lack of significant differences between DFU and non-DFU healing rates favors TPD’s broad applicability in diabetic wound management. Moreover, the absence of correlation between wound chronicity and healing time suggests that even long-standing wounds may respond to TPD, challenging assumptions about chronicity as a limiting factor.
Patients in the present study consistently reported reduced pain and improved comfort, likely due to TPD’s ability to mimic the structure and moisture content of healthy skin, which may reduce inflammation and nociceptive signaling.20 With dressing changes averaging every 10 days in the present study, TPD reduced disruption to the wound bed and eased the burden on patients and caregivers compared with daily gauze dressings.
From a health systems perspective, TPD offers practical advantages in both high- and low-resource settings. Although a unit cost of $164 per single-use blister (as of this writing) may be higher than gauze, reduced dressing frequency and associated labor can lower overall costs.21 In the United States, TPD is reimbursable under Healthcare Common Procedure Coding System code A2005.22 In middle-income countries where advanced technologies may not be available, TPD’s ease of storage, lack of refrigeration requirements, and simple application protocol enhance its appeal.23
In contrast, nonhealing DFUs are often referred for hyperbaric oxygen therapy, a time- and cost-intensive option, frequently exceeding $10,000 for a typical 40-session course.2,24,25 As of this writing, TPD costs $820 for up to 5 months of dressing changes, offering a more accessible and efficient alternative with fewer logistical barriers.21
These findings support TPD as a promising adjunct to diabetic wound care, particularly in settings where cost, adherence, or infrastructure pose challenges.
Limitations
This case series has several limitations. The retrospective design, small sample size, and lack of a control group limit causal inference and generalizability. Variability in wound types, anatomical sites, and comorbidities—as well as differences in adjunctive care, such as secondary dressings, off-loading, and debridement—introduce potential confounders. Clinical documentation was inconsistent across cases. Key variables such as hemoglobin A1c level, nutritional status, inflammatory markers, and microbiology were often missing, limiting analysis of systemic influences. Patient-reported outcomes were recorded qualitatively, and non-DFU wounds lacked standardized severity grading, which made comparisons challenging. Multivariable analysis was not feasible, and although no significant correlation was found between wound chronicity and healing time, this trend requires validation.
Conclusion
This multinational case series supports the clinical utility of TPD in managing complex diabetic lower extremity wounds. TPD was associated with consistent healing responses across a range of wound types and care settings, offering a practical, well-tolerated solution for patients for whom previous SOC had been unsuccessful. Its ability to reduce dressing change frequency while maintaining wound bed stability makes TPD especially valuable in settings where frequent follow-up or advanced adjuncts are limited.
These findings are clinically relevant for providers seeking accessible, efficient alternatives to traditional and high-cost advanced therapies. TPD’s unique formulation and ease of use may help streamline wound management protocols, improve patient comfort, and potentially reduce health care burden.
Further prospective, controlled studies involving larger and more diverse patient populations are needed to validate these findings, evaluate cost-effectiveness, and define optimal treatment protocols across various wound types and care environments. Taken together, the findings of the present case series support TPD as a versatile, biocompatible, and well-tolerated dressing that effectively addresses both the physiologic and logistical complexities of chronic wound management.
Author & Publication Information
Authors: Bradley A. Melnick, BS1,2; Shin Young Yu, BA1; Antoinette Nguyen, BA1,3; Jeewon Chon, MA1,4; Anmar Abu-Romman, MD1; Rahim Laiwalla, MS1,5; Joshua P. Weissman, MD1; Chitang J. Joshi, MD1; Tarifa Adam, BA1; and Robert D. Galiano, MD1
Affiliations: 1Division of Plastic Surgery, Department of Surgery, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; 2West Virginia School of Osteopathic Medicine, Lewisburg, WV, USA; 3University of Rochester School of Medicine and Dentistry, Rochester, NY, USA; 4Loyola University Chicago Stritch School of Medicine, Maywood, IL, USA; 5Chicago Medical School at Rosalind Franklin University of Medicine and Science, North Chicago, IL, USA
Acknowledgments: B.A.M. and S.Y.Y. contributed equally to this work.
Disclosure: Northwestern University and Robert Galiano, MD, receive research funding from Altrazeal Life Sciences Inc.
Ethical Approval: This study complied with the Declaration of Helsinki. Institutional review board approval was not required due to the retrospective study design and use of de-identified data.
Correspondence: Robert D. Galiano, MD; Division of Plastic Surgery, Department of Surgery, Northwestern University Feinberg School of Medicine, 259 E Erie St, Lavin 20-2060, Chicago, IL 60611; robert.galiano@nm.org
Manuscript Accepted: May 8, 2025
References
1. Milne T, Schoen D, Bower V, Burrows S, Westphal C, Gurr J. Healing time of diabetic foot ulcers: investigating the influence of infection and peripheral arterial disease. The Journal of Diabetic Foot Complications. 2013;5:29-38.
2. Hanley ME, Manna B. Hyperbaric Treatment of Diabetic Foot Ulcer. StatPearls. StatPearls Publishing LLC.; 2025.
3. Khanolkar MP, Bain SC, Stephens JW. The diabetic foot. QJM: An International Journal of Medicine. 2008;101(9):685-695. doi:10.1093/qjmed/hcn027
4. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367-2375. doi:10.1056/NEJMra1615439
5. Oliver TI, Mutluoglu M. Diabetic Foot Ulcer (Archived). StatPearls. StatPearls Publishing LLC; 2025.
6. Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153-165. doi:10.1111/nyas.13569
7. Kavitha KV, Tiwari S, Purandare VB, Khedkar S, Bhosale SS, Unnikrishnan AG. Choice of wound care in diabetic foot ulcer: a practical approach. World J Diabetes. 2014;5(4):546-56. doi:10.4239/wjd.v5.i4.546
8. Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle). 2014;3(8):511-529. doi:10.1089/wound.2012.0401
9. Hoffstad O, Mitra N, Walsh J, Margolis DJ. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38(10):1852-1857. doi:10.2337/dc15-0536
10. Meloni M, Izzo V, Giurato L, Lázaro-Martínez JL, Uccioli L. Prevalence, clinical aspects and outcomes in a large cohort of persons with diabetic foot disease: comparison between neuropathic and ischemic ulcers. J Clin Med. 2020;9(6):1780. doi:10.3390/jcm9061780
11. Bohn G. Transforming powder dressing combined with total contact cast may shorten days to heal Wagner Grade 2 neuropathic diabetic foot ulcers. Journal of Wound Ostomy and Continence Nursing. 2010;37(3).
12. Smith SP. Use of a transforming powder dressing in the lower leg wounds of two older patients: case studies. J Wound Care. 2019;28(Sup7):S40-S43. doi:10.12968/jowc.2019.28.Sup7.S40
13. O‘Connor MJ, Ho KC, Sriram N, et al. A systematic review on the use of transforming powder dressing for wound care. Int Wound J. 2024;21(9):e70046. doi:10.1111/iwj.70046
14. Fitzgerald RH, Bharara M, Mills JL, Armstrong DG. Use of a Nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6(2):133-9. doi:10.1111/j.1742-481X.2009.00596.x
15. Forstner C, Leitgeb J, Schuster R, et al. Bacterial growth kinetics under a novel flexible methacrylate dressing serving as a drug delivery vehicle for antiseptics. Int J Mol Sci. 2013;14(5):10582-10590.
16. Coye TL, Bargas Ochoa M, Zulbaran-Rojas A, et al. Healing of diabetic neuropathic foot ulcers receiving standard treatment in randomised controlled trials: a random effects meta-analysis. Wound Repair Regen. 2025;33(1):e13237-n/a. doi:10.1111/wrr.13237
17. Armstrong DG, Orgill DP, Galiano R, et al. A multicenter, randomized controlled clinical trial evaluating the effects of a novel autologous heterogeneous skin construct in the treatment of Wagner one diabetic foot ulcers: final analysis. Int Wound J. 2023;20(10):4083-4096. doi:10.1111/iwj.14301
18. Edmonds M, Manu C, Vas P. The current burden of diabetic foot disease. J Clin Orthop Trauma. 2021;17:88-93. doi:10.1016/j.jcot.2021.01.017
19. Game FL, Hinchliffe RJ, Apelqvist J, et al. A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2012;28(S1):119-141. doi:10.1002/dmrr.2246
20. Assadian O, Arnoldo B, Purdue G, et al. A prospective, randomised study of a novel transforming methacrylate dressing compared with a silver-containing sodium carboxymethylcellulose dressing on partial-thickness skin graft donor sites in burn patients. Int Wound J. 2015;12(3):351-6. doi:10.1111/iwj.12136
21. Altrazeal® Transforming Powder Dressing. Altrazeal Life Sciences Inc. https://altrazeal.com/product/altrazeal-transforming-powder-dressing/
22. CMS Manual System Pub 100-04 Medicare Claims Processing Page 43 (2025).
23. Safety Data Sheet, Altrazeal Transforming Powder Dressing. Uluru, Inc.; 2015. https://www.medline.com/media/catalog/Docs/MSDS/MSD_SDSD94388.pdf
24. Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17(5):1-142.
25. Ennis WJ, Huang ET, Gordon H. Impact of hyperbaric oxygen on more advanced Wagner grades 3 and 4 diabetic foot ulcers: matching therapy to specific wound conditions. Adv Wound Care (New Rochelle). 2018;7(12):397-407. doi:10.1089/wound.2018.0855
















