A Precision-Based Approach for Bioactive Skin Allograft Application in Nonhealing Wounds Using Bacterial Fluorescence Imaging
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. Chronic wounds present significant clinical challenges and often require advanced modalities such as cellular and/or tissue-based products (CTPs), also referred to as cellular, acellular, and matrix-like products. Bioactive skin allograft (BSA) is a type of CTP composed of living fibroblasts, keratinocytes, cytokines, and collagen that is widely used for nonhealing wounds. Before applying a BSA, standard of care involves lowering the bioburden through wound bed preparation; however, classic methods to assess bioburden can be unreliable. Bacterial fluorescence imaging (FL-imaging) is a recent technology that allows for the detection of clinically significant bioburden using a noninvasive, point-of-care device. Few studies have applied FL-imaging directly to BSA management. Objective. To evaluate the use of FL-imaging to direct and manage the use of BSAs before, during, and after their application. Materials and Methods. BSAs were applied after ensuring adequate tissue perfusion, 30 days of local wound care, and medical optimization. Debridement was performed and adequacy verified using FL-imaging before BSA placement. FL-imaging was used to monitor graft sites at day 3, 7, and 14 after initial application. Results. Although FL-imaging confirmed initial debridement success, subsequent scans revealed bioburden recurrence that compromised BSA viability in several cases. These findings informed the development of a preliminary protocol using FL-imaging to confirm adequate debridement and guide postoperative graft monitoring and salvage. Conclusion. The systematic adoption of FL-imaging could provide a consistent method for wound bed preparation and graft monitoring in the future, while encouraging clinicians to use BSAs with a cost-efficient, consistent, and evidence-based approach.
Cellular and/or tissue-based products (CTPs)—also referred to as skin substitutes, or cellular, acellular, and matrix-like products—represent a category of therapeutic modalities designed to address underlying tissue repair deficiencies by promoting angiogenesis, cellular proliferation and migration, extracellular matrix (ECM) remodeling, and immune modulation.1,2 One such product is a cadaver-harvested bioactive skin allograft (BSA). BSA is a cryopreserved, human split-thickness allograft with a native ECM composition that contains living fibroblasts, keratinocytes, cytokines, and collagen. BSAs were among the first skin substitutes developed and have demonstrated effectiveness in treating chronic diabetic foot ulcers and venous leg ulcers compared with standard care.3-5 Despite dozens of commercially available products approved for clinical use, protocols to guide their selection, use, and monitoring are not well-defined. As a result, the selection, utilization, and management of these advanced wound care modalities are mainly based on anecdotal clinician preference, case studies, and expert opinion consensus articles.6
A current consensus for CTP use is to reduce the bacterial load and remove nonviable tissue before application.7 Bacterial colonization and infection are critical considerations because pathogen type, quantity, and biofilm presence can significantly impair the healing process.8,9 The association between delayed healing rates and elevated bacterial loads has been documented for decades and is well established.10 Clinicians can discern the presence of bioburden and local infection by visualizing the wound bed by assessing the clinical signs and symptoms (CSS) of infection, but this method is subjective and often inaccurate.11 Culture-based methods are used to identify wound pathogens to provide critical information to guide specific treatments. When used alone, however, these methods often lack precision regarding location within the wound bed, may miss biofilms and anaerobes, are time-consuming, and offer limited predictive value for healing.9,12 Without directly actionable and reliable methods to assess wound bioburden and demonstrate a clean wound bed, the placement and subsequent monitoring of BSAs has remained largely unstandardized.
The recent development of bacterial fluorescence imaging (FL-imaging) has provided an opportunity to fill this gap. FL-imaging is a reproducible, practical imaging method to quantify bioburden that uses violet light (405-nm wavelength) to detect elevated bioburden on the wound surface at a threshold of 104 CFU/g, providing clinicians with a novel visual assessment of elevated bioburden.13 In addition to more accurately detecting elevated bioburden compared with CSS alone, FL-imaging has been shown to improve overall healing outcomes.11,14 Using the information provided by FL-imaging, wound treatment decisions, including debridement, antiseptics, and antibiotic choice, can be more objectively tailored.
In the present clinical case series, FL-imaging actively guided the use of BSAs in treating chronic wounds by identifying graft susceptibility to bacterial colonization and infection.
Materials and Methods
For each case, BSAs (TheraSkin; LifeNet Health) were utilized after establishing adequate tissue perfusion, applying 30 days of local wound care measures, and optimizing medical management. Immediately prior to BSA application, a thorough surgical debridement was performed using both an ultrasonic debridement device (SonicOne O.R.; Bioventus) and a 3-mm ringed curette. Adhesive skin closure strips were used for surgical fixation of the grafts, and cadexomer iodine pads were placed postoperatively over all grafts. FL-imaging (MolecuLight Dx; MolecuLight) was used for wound imaging following debridement and at subsequent follow-up visits.
Results
Case 1
Case 1 was a 44-year-old male with systemic lupus erythematosus (SLE), hypertension, and chronic deep vein thrombosis in the left popliteal vein. He presented to the clinic with an atypical left lateral ankle ulcer that had developed during an SLE flare. Initial treatment included mycophenolate mofetil, warfarin, and 81 mg aspirin. Due to a lack of healing progression, the patient underwent BSA grafting 3 months after failure of initial treatment. FL-imaging was unavailable at the time of the initial debridement. At the time, a healthy-appearing, clean wound bed on visual inspection was the standard approach to determining adequate wound bed preparation.
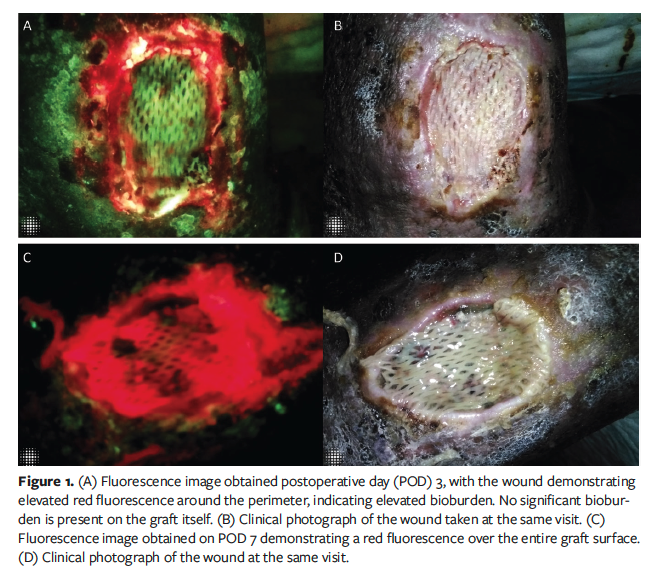
FL-imaging became available to the clinic for routine use during the course of this treatment, prompting exploration into its potential benefit for evaluating graft constructs post-application. FL-imaging was performed 3 days after grafting. On visual inspection, there were no clinical signs of infection; however, FL-imaging detected elevated bioburden at the wound perimeter (Figure 1A, 1B). The wound was irrigated with saline and re-dressed with a fresh cadexomer iodine pad to further control the periwound bioburden. Seven days post-procedure, FL-imaging revealed elevated bioburden over the entire graft surface (Figure 1C, 1D). However, because the cadaver graft remained securely adherent to the wound bed, it was left in place. FL-imaging was repeated at a 2-week follow-up appointment, demonstrating persistent bacterial colonization of the graft. The construct had become nonadherent to the wound bed, necessitating its removal and subsequent debridement of the wound bed. Treatment with 2 additional BSAs was attempted but failed due to recurrent bioburden. The wound healed with continuation of local dressings and use of energy-based modalities.
Case 2
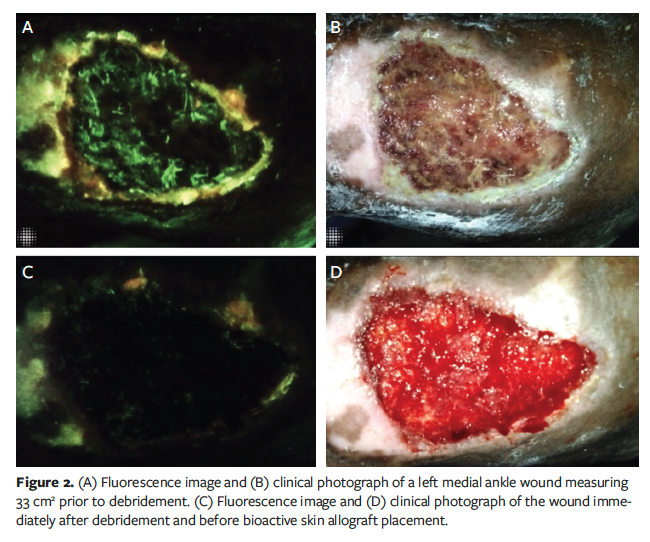
Case 2 was a 66-year-old female with a left medial ankle venous ulcer wound 33 cm2 in size that was surgically debrided and treated with a BSA. Given previous experience, FL-imaging was performed prior to graft placement to confirm adequate debridement (Figure 2). Three days post-procedure, repeat FL-imaging was unremarkable. One week post-
procedure, FL-imaging detected elevated bioburden on the wound perimeter and overlapping portions of the graft (Figure 3A). Colonized sections of the graft were sharply excised, and the graft was treated with a hypochlorous wound solution soak for 10 minutes.
Two weeks later, repeat FL-imaging showed absence of bioburden (Figure 3B). Over a 12-week treatment period, 2 additional grafts were applied, reducing the wound size to 9.36 cm². No further grafts were necessary, and complete wound closure was achieved with conservative management within 6 months (Figure 3C).
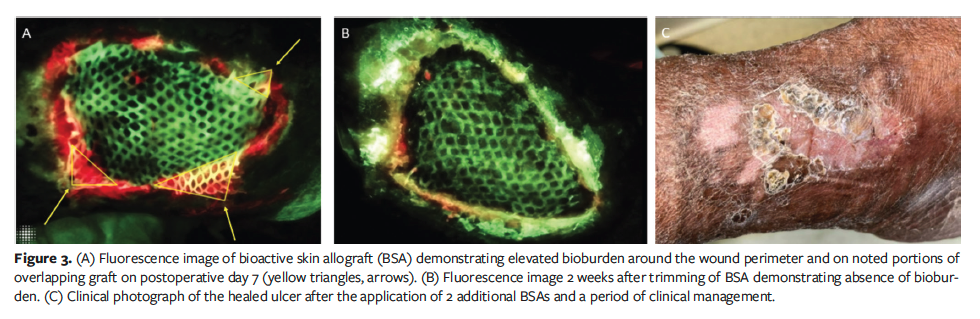
Case 3
Case 3 was a 90-year-old male with a history of venous disease currently undergoing the FL-imaging for BSAs protocol for a left medial ankle venous ulcer initially measuring 17.8 cm2 (Figure 4A). Over a 3-month period, the patient was treated with 2 BSAs, resulting in a 20.3% reduction in wound area. FL-imaging did not detect elevated bioburden on the graft or wound perimeter at follow-up visits (Figures 4B, 4C). Consequently, no excision of the graft was necessary, and the grafts were re-dressed with cadexomer iodine pads. Further BSA applications were deferred, and local wound care measures were continued. Over the subsequent 2.5 months, the wound contracted significantly, achieving a total 75.8% reduction in area and measuring 4.3 cm² (Figure 4D).
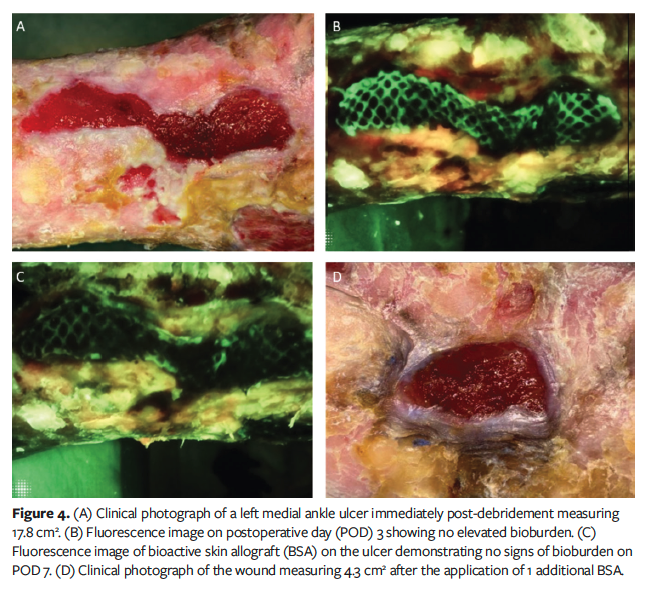
Case 4
Case 4 was a 39-year-old male who was treated with BSA for a nonhealing abdominal wound 268.8 cm2 in size after multiple exploratory laparotomies and bowel resections (Figure 5A). The patient had adherent bowel immediately underneath the wound surface, precluding aggressive surgical debridement efforts. He underwent an ultrasonic debridement and BSA grafting after FL-imaging demonstrated a clean bed post-debridement. On post-procedure day 3, FL-imaging showed no evidence of elevated bioburden. However, an odor was noted on post-procedure day 7, and FL-imaging demonstrated elevated bioburden at the perimeter of the wound. Using real-time FL-imaging, fluorescent areas of the graft were excised with scissors (Figure 5B). The wound was irrigated, and new cadexomer iodine pads were placed. At 10 days post-BSA placement, FL-imaging revealed increased levels of red fluorescence. Additional targeted debridement of fluorescent graft was conducted to prevent the progression of graft colonization. Only viable graft tissue that remained adherent to the wound bed was preserved (Figure 5C).
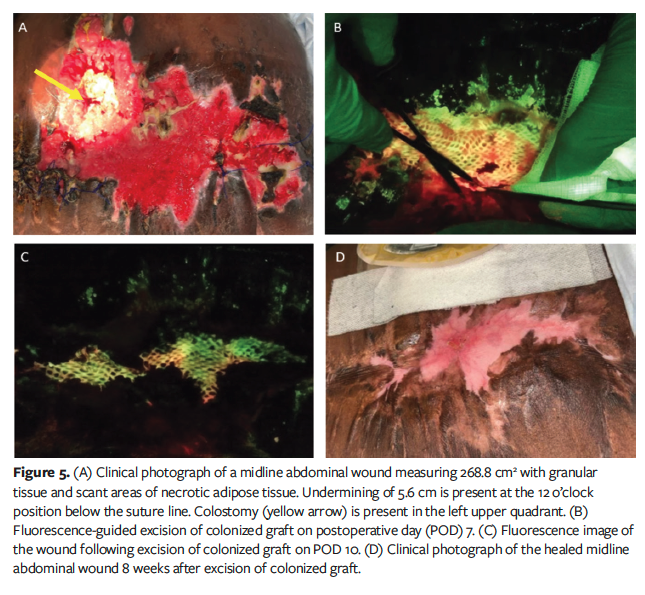
Five weeks after the FL-imaging–
guided excision, the wound measured 33.3 cm2 (88% area reduction), with healthy granulation tissue forming at the edges where epithelial migration had occurred. FL-imaging revealed no signs of elevated bioburden. The patient was transitioned to regular dressing changes. No further BSAs were required, and the wound ultimately healed 8 weeks after excision of the graft without other infectious complications (Figure 5D). Total time to healing was 4.5 months.
Discussion
Elevated bacterial loads hinder wound healing and impair natural responses to tissue injury, making bioburden management a cornerstone in wound diagnosis and treatment.10 Concerning the use of BSAs to accelerate the healing of stalled wounds, data on the optimal duration to leave a BSA on the wound bed are limited and vary based on clinician preference, resulting in an inconsistent, subjective use of the technology. The current case series illustrates how FL-imaging identified compromised BSA grafts and reframed clinical decision-making regarding the appropriateness, duration, and quantity of BSA applications.
BSAs are designed to be deployed when a wound does not exhibit healing after 30 days of standard wound care. With the advent of FL-imaging, there has been a consensus on its practical utility to verify that adequate wound bed preparation can be achieved.7,15 Recent work in burn wounds has supported this concept by demonstrating that positive FL-imaging before split-thickness skin grafting was predictive of graft failure.16
Less attention has been paid in the literature to the management of BSAs following application. The cases in the current report illustrate how bioburden can continue to develop on BSAs after initial placement, even if pre-placement debridement appears successful. In the cases presented in the current series, follow-up FL-imaging detected elevated bioburden 3 days to 7 days after graft placement, despite adequate debridement and wound bed preparation. Notably, bioburden often emerged first around the periwound, suggesting that adjacent periwound skin may serve as a reservoir for bacterial colonization, especially in the presence of a BSA. It is possible that while debridement helped remove surface-level bioburden, it may have been insufficient in eradicating biofilm infection, resulting in microcolony establishment in deeper tissues.17 While FL-imaging did not immediately identify significant bioburden post-debridement, deeper microcolonies and biofilms, undetectable by either FL-imaging’s 1.5-mm tissue penetration depth or the 104 CFU/g threshold, might have contributed to recalcitrant infection and colonization of subsequent grafts.
One factor complicating graft success for Case 1 was the immunosuppressive therapy the patient was receiving for SLE. Although essential for preventing graft rejection in transplant patients and chronic autoimmune conditions, long-term immunosuppression can increase the risk of infection and is known to impair wound healing.18,19 The infectious diseases community describes this as a “net state of immunosuppression,” highlighting the challenges of infection prevention in such patients.20 This concept may also apply to BSA use. Unlike split-thickness skin autografts, BSAs are temporary because they are cadaver-harvested, but BSAs may persist longer in immunosuppressed patients due to reduced likelihood of allograft rejection. Simultaneously, immunosuppression can mask clinical signs of infection, making FL-imaging particularly valuable in detecting subclinical bioburden in the absence of CSS of infection. Given these factors, heightened vigilance and serial FL-imaging postoperatively would be especially helpful for optimizing graft outcomes in immunosuppressed patients.
While cryopreserved BSAs were the product of choice for the patients in this case series, clinicians have access to a range of skin substitutes, each with unique characteristics and distinct application protocols. Different skin substitute products may vary in their susceptibility to bacterial colonization based on their composition and intended duration of use. For example, bioactive grafts or those designed to remain in place for extended periods may be less tolerant compared with acellular materials or products intended for more frequent reapplication. Manufacturer recommendations for cryopreserved BSAs suggest reapplication every 2 weeks,21 but real-world practice varies based on clinician discretion and clinical response to therapy. A large cohort study of BSAs used to treat full-thickness injuries reported that an average (SD) of 4.24 (2.80) grafts over a mean 15.38 (9.54) weeks were required to achieve wound closure, with application intervals ranging from once weekly to every 6 weeks.3 The authors of that study note that the time between applications was determined by the clinician’s assessment of wound progression and the clinical appearance of graft take.3 The incorporation of FL-imaging into this decision-making schema would be of benefit to clinicians when deciding on the viability and cadence of future grafts.
The cases in the current report also provide strategies for situations in which elevated bioburden threatens the success of BSAs. Certain strategies, such as the use of antimicrobial primary dressings, should be used prophylactically immediately following placement. If bioburden becomes a recurrent problem, FL-imaging can be used in tandem with wound cultures by guiding clinicians to more accurately sample locations, increasing diagnostic yield and mitigating against antibiotic misuse.22-24 Alternative strategies may involve applying antiseptic soaks to the BSA or using hypochlorous solution to treat the colonized periwound in cases of heavy bioburden. Novel approaches to managing bioburden in BSA treatment, such as selectively excising areas of a graft with high bacterial loads while preserving healthy tissue, are not well-explored and warrant further study. The only previous mention of such attempts was documented in a 2019 editorial, which stated that cyan fluorescence was detected by FL-imaging on a biodegradable temporizing matrix and was successfully debrided away without further colonization.25 In the current case series, in both Case 2 and Case 4, by identifying and sharply excising localized areas of bioburden before the entire graft was compromised, local sources of infection were eliminated and the need for additional grafting was avoided. Thus, the authors of the current series propose the term “BSA salvage” to describe interventional approaches to preserve the longevity of BSAs (Figure 6). The authors also suggest that BSA salvage should only be attempted in cases in which the BSAs are still partially viable and adherent to the wound surface.
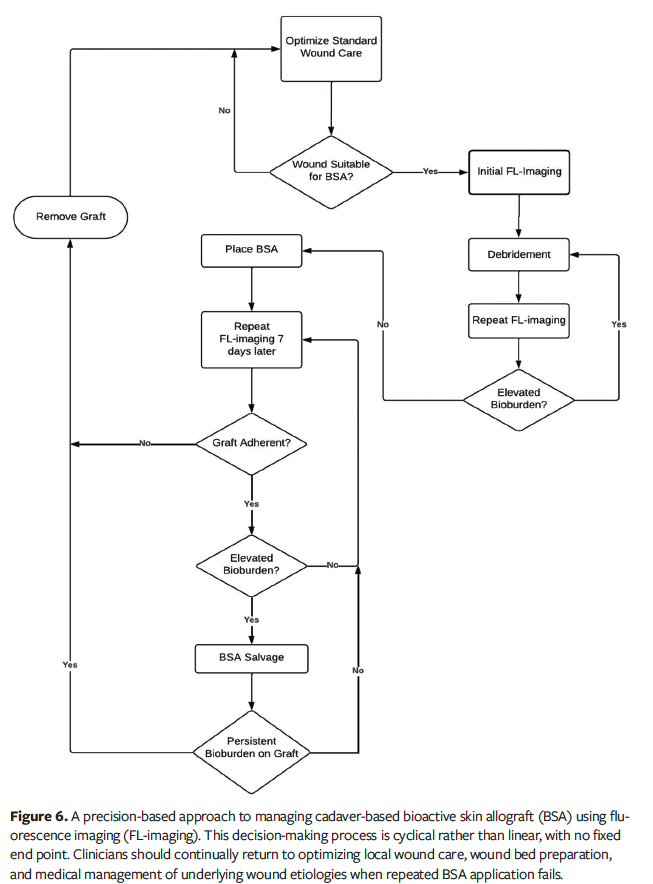
Limitations
Several limitations should be noted. While FL-imaging demonstrates high specificity for the effect of elevated bioburden on the likelihood of graft failure, the intrinsic 104 CFU/g of tissue detection threshold may endow the diagnostic with limited sensitivity. Additionally, as a small case series, the findings are descriptive and hypothesis-generating rather than conclusive. Finally, the study was conducted in a single clinical setting with a limited number of patients, which may introduce selection bias and is not representative of practice patterns in other centers. For example, independent clinical decision-making and patient comorbidities might have influenced wound healing outcomes independently of BSA use. Patient inclusion criteria were intentionally broad, encompassing individuals with nonhealing wounds regardless of etiology. While this heterogeneity may limit internal validity, it reflects real-world clinical scenarios and supports the broader applicability of FL-imaging for postoperative BSA monitoring.
Conclusion
There is a pressing need for objective indicators to determine how elevated bioburden develops and influences treatment outcomes. The findings of the current series suggest that FL-imaging is useful as a negative predictor that identifies when elevated bioburden can impede BSA success, rather than as a definitive indicator of readiness. While the presence of fluorescence prior to application was associated with subsequent graft failure, the absence of fluorescence did not reliably predict successful graft take. Future prospective studies should evaluate the relationship between FL-imaging and BSA treatment success, BSA duration, wound area reduction, and healing outcomes. Ultimately, FL-imaging should be incorporated as an immediate and noninvasive indicator of bioburden to facilitate a more objective and systematic approach to using BSAs in treating chronic wounds.
Author and Public Information
Authors: Jack L. Knott, BS; Kathy K. Wang, MD; Daniel P. deLahunta, MD; Duncan MacIntyre, BS; and William J. Ennis, DO
Affiliations: Section of Wound Healing and Tissue Repair, Department of Surgery, University of Illinois College of Medicine, Chicago, IL, USA
Disclosure: W.J.E. is a consultant for Healogics, LLC. All other authors disclose no conflicts of interest.
Ethical Approval: Informed consent was provided by the patients to publish the details of the subjects’ clinical cases and the associated images.
Correspondence: Jack L. Knott, BS, 820 S. Wood St., Suite 376, CSN (M/C) 958, Chicago, IL 60612; jknott24@uic.edu
Manuscript Accepted: July 17, 2025
References
1. Holl J, Kowalewski C, Zimek Z, et al. Chronic diabetic wounds and their treatment with skin substitutes. Cells. 2021;10(3):655. doi:10.3390/cells10030655
2. Kondej K, Zawrzykraj M, Czerwiec K, Deptuła M, Tymińska A, Pikuła M. Bioengineering skin substitutes for wound management—perspectives and challenges. Int J Mol Sci. 2024;25(7):3702. doi:10.3390/ijms25073702
3. Flood MS, Weeks B, Anaeme KO, et al. Treatment of deep full-thickness wounds containing exposed muscle, tendon, and/or bone using a bioactive human skin allograft: a large cohort case series. Wounds. 2020;32(6):164–173.
4. Kirsner RS, Margolis D, Masturzo A, Bakewell K. A real-world experience with the bioactive human split thickness skin allograft for venous leg ulcers. Wound Repair Regen. 2020;28(4):547–552. doi:10.1111/wrr.12818
5. Armstrong DG, Galiano RD, Orgill DP, et al. Multi-centre prospective randomised controlled clinical trial to evaluate a bioactive split thickness skin allograft vs standard of care in the treatment of diabetic foot ulcers. Int Wound J. 2022;19(4):932–944. doi:10.1111/iwj.13759
6. Wu S, Carter M, Cole W, et al. Best practice for wound repair and regeneration use of cellular, acellular and matrix-like products (CAMPs). J Wound Care. 2023;32(Sup4b):S1–S31. doi:10.12968/jowc.2023.32.Sup4b.S1
7. Serena TE, Harding K, Queen D. Point-of-care fluorescence imaging to optimise wound bed preparation prior to cellular and/or tissue-based product (CTP) application. Int Wound J. 2023;20(9):3441–3442. doi:10.1111/iwj.14446
8. Yang Y, Huang J, Zeng A, Long X, Yu N, Wang X. The role of the skin microbiome in wound healing. Burns Trauma. 2024;12:tkad059. doi:10.1093/burnst/tkad059
9. White EK, Grice EA. The wound microbiome. Cold Spring Harb Perspect Biol. 2023;15(6):a041218. doi:10.1101/cshperspect.a041218
10. Uberoi A, McCready-Vangi A, Grice EA. The wound microbiota: microbial mechanisms of