The Impact of an Aggressive Clinic-Based Diabetic Foot Protocol: A Single-Center Case Series
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. The considerable health and economic burden of hard-to-heal wounds has become increasingly prominent. Diabetic foot ulcers (DFUs), as a representative type of these wounds, pose substantial challenges for health care professionals in both treatment and long-term management. Objective. To evaluate the efficacy of a standardized wound hygiene protocol in promoting healing outcomes for DFUs and to provide supplementary clinical evidence to inform wound care practices. Materials and Methods. Diabetic foot care specialists administered a wound hygiene protocol to 20 patients with DFUs. The protocol comprised 4 key components: cleansing, debridement, wound edge refashioning, and dressing coverage. Wound healing progress was monitored and documented over a 12-week observation period. Results. After 12 weeks of treatment with the wound hygiene protocol in the multidisciplinary diabetic foot clinic, 16 of the 20 patients achieved complete wound healing, while the remaining 4 patients showed significant wound improvement, resulting in a healing rate of 80%. Wound area, exudate level, and pain scores all showed significant improvement compared to baseline, with statistically significant differences (P < .001). The overall mean (SD) wound area reduction rate was 95.11% (10.46%), with a mean healing rate of 1.32 (0.36) cm² per week. The average healing time for patients with complete healing was 56.44 (25.12) days. Conclusion. This case series demonstrates that the implementation of a wound hygiene protocol significantly enhances healing outcomes in patients with DFUs. These findings provide critical insights for the management of diverse hard-to-heal wounds in clinical practice.
Introduction
The substantial health and economic burden caused by hard-to-heal wounds is becoming increasingly evident worldwide. In the United States alone, annual Medicare expenditures on wound care are estimated to range from $28.1 billion to $96.8 billion.1 Based on research into hard-to-heal wounds and clinical experience in wound care practice, Murphy et al2 introduced the strategy of wound hygiene in an international consensus document on wound hygiene published in 2020, titled “Defying hard-to-heal wounds with an early antibiofilm intervention strategy: wound hygiene.” The wound hygiene protocol is intended to support the early and repeated management of biofilms, thereby addressing an underlying cause of chronic, nonhealing wounds. The protocol comprises 4 key components: cleansing, debridement, wound edge refashioning, and dressing application. Its core principle is the removal or minimization of all harmful substances within the wound, the prevention of their re-formation, and the maintenance of a clean wound environment to initiate and sustain the healing process. Therefore, it is reasonable to hypothesize that clinical implementation of the 4-step wound hygiene protocol might promote wound healing in DFUs and improve patients’ quality of life.
The present study aimed to evaluate the efficacy of a wound hygiene protocol in the management of DFUs. The primary outcome measures were the wound area reduction rate and time to healing (in days), while secondary outcomes included wound healing rate, exudate level, and wound pain scores.
Materials and Methods
Patient recruitment
A total of 20 eligible patients were recruited in the clinical setting. The inclusion criteria were as follows: age 18 years or older; diagnosis of diabetic foot according to the Chinese Guidelines for the Diagnosis and Treatment of Diabetic Foot3; DFUs classified as Wagner grade I, II, or III4; a history of nonhealing or worsening wounds following prior treatment at other medical institutions; and the presence of at least 1 well-perfused pedal artery confirmed by either Doppler ultrasound or transcutaneous oxygen pressure greater than or equal to 30 mm Hg. Exclusion criteria included ulcers located above the ankle joint, ongoing radiotherapy or chemotherapy, and the presence of life-threatening systemic infection or significant cognitive impairment.
Wound assessment and treatment
All enrolled patients received wound care in accordance with the 4-step wound hygiene protocol,5 which was administered 3 times per week by specialized diabetic foot care nurses in the outpatient clinic. Data collection and follow-up were conducted over 12 weeks. Before each treatment session, wounds were comprehensively assessed, photographed, and evaluated using standardized wound parameters to monitor healing progress. The standardized wound hygiene protocol was systematically implemented through 4 sequential phases to ensure consistent and effective wound management.
First, the wound and periwound skin were gently cleansed using povidone-iodine gauze, followed by thorough irrigation with normal saline to remove scabs, devitalized tissue, and wound debris. Second, sharp debridement was performed according to the wound characteristics and the patient’s pain tolerance, with the goal of thoroughly eliminating necrotic tissue, slough, and nonviable material. Following publication of the aforementioned consensus document on wound hygiene,2 the diabetic foot care specialists consistently used a standardized sharp debridement technique. Using sterile instruments such as scissors or forceps, they removed slough and necrotic tissue to create a wound environment conducive to tissue regeneration. Each patient underwent debridement 1 to 2 times per week. Third, wound edge refashioning was meticulously conducted by excising devitalized tissue and excessive hyperkeratosis along the wound margins to optimize edge-to-bed alignment and facilitate epithelial migration and tissue regeneration. Finally, an antimicrobial dressing composed of carboxymethyl cellulose sodium-silver (Aquacel Ag+ Extra; Convatec) was applied to suppress residual biofilm and prevent its re-formation, followed by an outer layer of breathable cotton gauze to provide mechanical protection. Throughout the 12-week intervention period, any adverse events—such as ulcer progression, systemic infection, or other wound-related complications—were actively monitored and documented.
During the study period, demographic characteristics and wound-related parameters of all participants were systematically collected. Baseline demographic data included age, sex, education level, activities of daily living (assessed using the Barthel Index), residency status, and duration of diabetes.
Wound-specific parameters included the duration of DFU, Wagner grade, wound location, wound area, exudate level, pain scores, and wound pH (measured using wound surface pH test strips). The level of wound pain was recorded according to the 10-point Visual Analog Scale (VAS), with 0 being “no pain” and 10 being “worst pain possible.”
All wound assessments were conducted by diabetic foot care specialists. In addition, digital photographs of the wound were taken at each patient visit to allow for visual comparison and to assess healing progress. This comprehensive data collection provided a solid foundation for the subsequent analysis of treatment outcomes and the clinical evaluation of the wound hygiene protocol’s effectiveness.
Systemic management
In addition to local wound care, all patients received systemic interventions during the treatment period. Glycemic control was overseen by endocrinologists, who adjusted antidiabetic medications based on regular blood glucose monitoring. Nutritional support was provided by dietitians through individualized dietary counseling, focusing on optimizing metabolic health and promoting wound healing. These systemic measures were implemented alongside wound hygiene care to support overall recovery.
Monitoring of wound healing
As noted above, the primary outcomes were (1) the wound area reduction rate (percentage decrease from baseline) and (2) the time to complete healing, defined as the number of days required to achieve full epithelialization with resolution of exudate. Secondary outcomes included: (1) wound pain scores (assessed using the VAS), (2) wound exudate level, and (3) wound healing rate.
Time to healing was defined as the number of days needed for complete wound closure, characterized by full epithelialization and the absence of exudate. If complete healing occurred at any point during the treatment period, the time to healing was recorded accordingly. If the ulcer remained unhealed after 12 weeks of treatment, then the trial was considered terminated, and the final wound assessment was documented as the study end point.
Ethical approval and patient consent
Written informed consent was obtained from all enrolled patients, explicitly authorizing both study participation and the publication of potentially identifiable clinical details with accompanying wound images. The ethical approval for this study was obtained from the Ethics Committee of Huadong Hospital, Fudan University, Shanghai, China (Approval No. 2024K262).
Results
A total of 20 patients were enrolled in this case series study, comprising 11 males (55%) and 9 females (45%). The patients’ ages ranged from 44 years to 84 years, with a mean age of 66 years. The duration of diabetes varied from 3 years to 40 years, with an average (SD) duration of 23.20 (12.28) years. The demographic characteristics of the patients are presented in Table 1. Representative wound healing trajectories from 3 prototypical cases are illustrated in Figures 1-3.
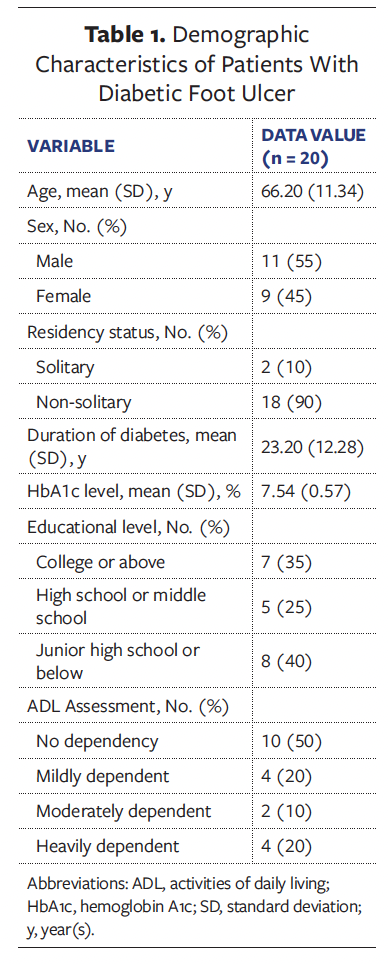
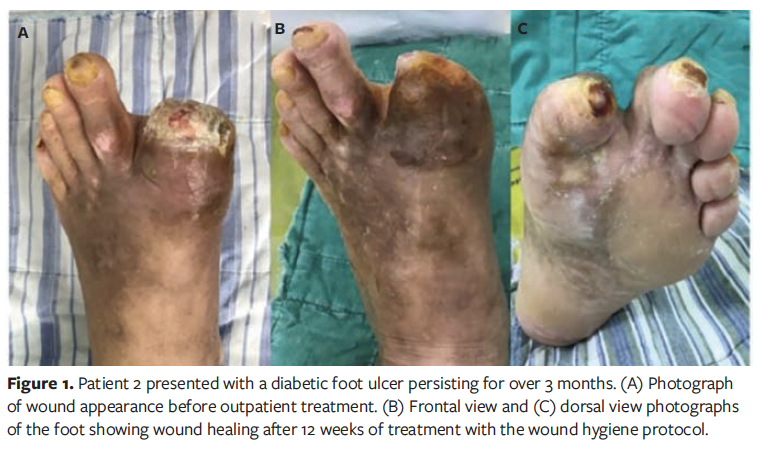
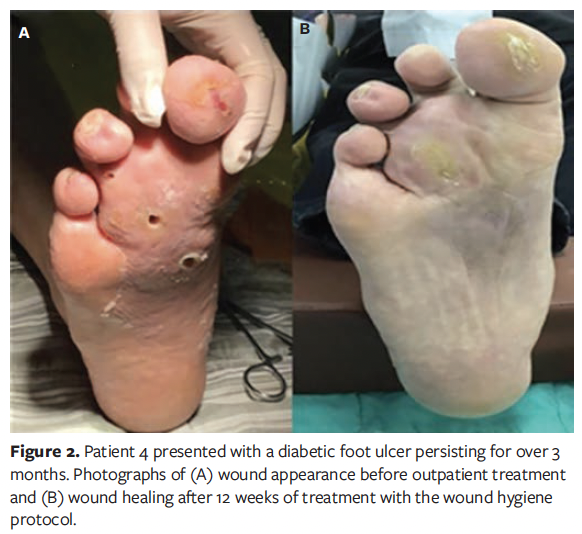
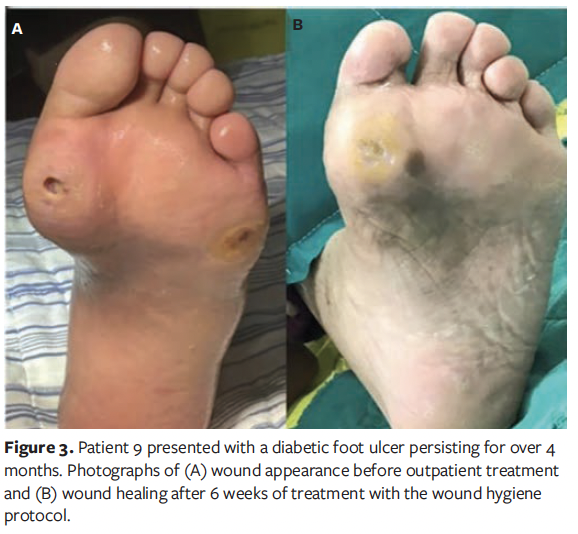
The mean (SD) duration of DFU in all patients was 41.45 (26.87) days, with an average wound area of 11.36 (5.27) cm² and a mean pain score of 3.45 (1.28). The wound pH averaged 7.85 (0.38), consistently maintaining alkalinity. According to the Wagner classification system, 13 patients (65.0%) were classified as grade II and 7 patients (35.0%) as grade III. Regarding wound location, in 11 patients (55.0%) the wound was located on the plantar surface of the foot, in 5 patients (25.0%) it was located on the dorsal surface of the foot, and in 4 patients (20.0%) it was located on the toes. A summary of baseline wound characteristics in these patients with DFU is presented in Table 2.
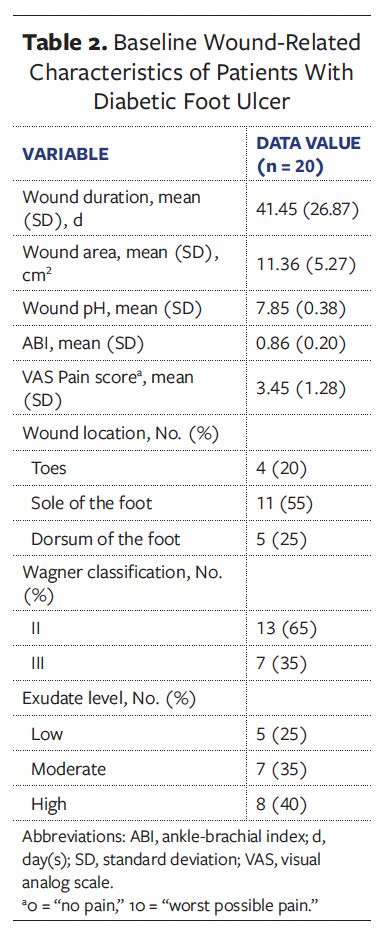
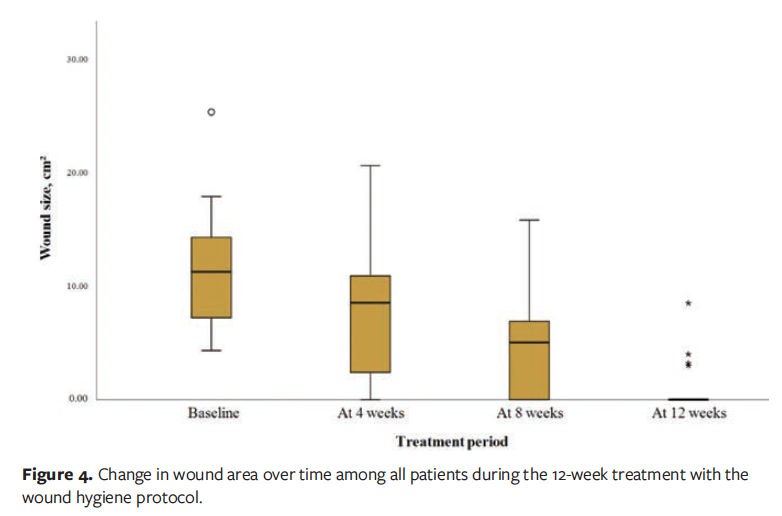
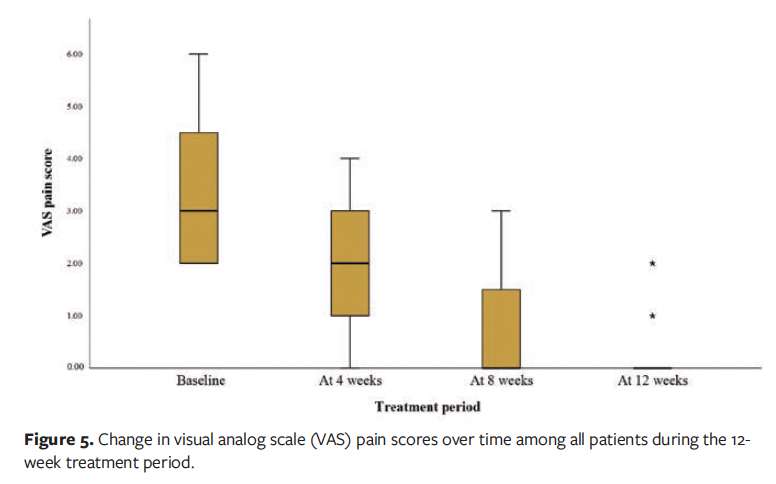
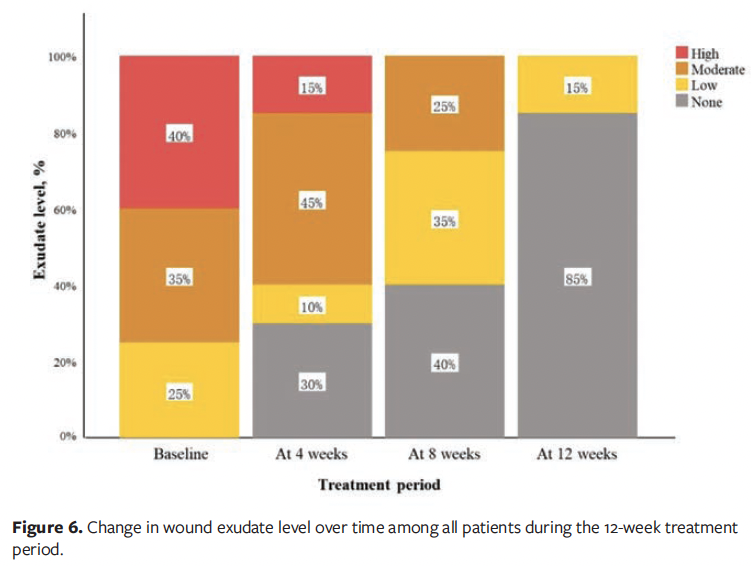
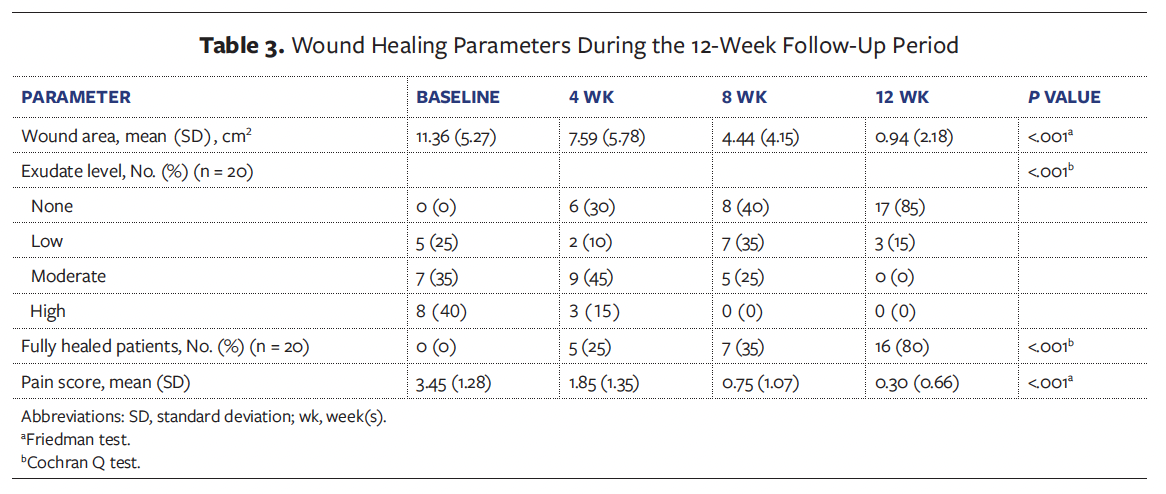
At the 12-week follow-up visit, complete wound healing was achieved in 16 of the 20 patients (80.0%), while the remaining 4 patients (20.0%) demonstrated clinically significant improvement (≥50% wound area reduction). The mean (SD) wound area reduction rate was 95.11% (10.46%), corresponding to an average healing rate of 1.32 (0.36) cm² per week. Among patients who achieved complete epithelialization, the mean time to wound closure was 56.44 (25.12) days. Longitudinal analysis revealed statistically significant improvements in healing outcomes (P < .001), including progressive reductions in wound area (Figure 4), pain scores (Figure 5), and exudate level (Figure 6) relative to baseline (Table 3).
Discussion
In this case series study, diabetic foot care specialists implemented a 12-week wound hygiene protocol for patients with DFU. The results demonstrated significant reductions in wound area, exudate level, and pain scores, with statistically significant differences compared with baseline (P < .001). The mean (SD) wound area decreased from 11.36 cm² at baseline to 0.94 cm² at week 12. All patients showed complete wound healing or marked improvement, with a healing rate of 80%. These findings suggest that the wound hygiene protocol is effective in promoting DFU healing, which is consistent with previous clinical studies reporting that regular implementation of wound hygiene measures can facilitate the transition of chronic wounds toward a healing trajectory.6
It is well established that hyperglycemia-induced oxidative stress is a key factor contributing to delayed wound healing in patients with diabetes.7 In the present case series, in addition to local wound care, patients received glycemic control and nutritional support provided by endocrinologists and dietitians. Furthermore, during the 12-week study period, for 55% of wounds located on the plantar surface, diabetic foot care specialists used felted foam dressings as an off-loading strategy and advised patients to minimize weightbearing activities. Off-loading therapy is critical for the healing of DFUs.8 Notably, no adverse events—such as complications, hospitalizations, amputations, or deaths—were observed during the present study. To the best of the knowledge of the current authors, this is among the first studies to report the clinical effectiveness of a wound hygiene protocol in the treatment of DFUs within an Asian population.
DFUs have emerged as a significant health concern, severely affecting the quality of life of individuals with diabetes. Although the authors of the present study observed the beneficial effects of standardized wound hygiene protocols on DFU management, there remains a critical shortage of health care professionals trained to implement wound hygiene in clinical practice. This highlights the urgent need for comprehensive wound hygiene education and skill development programs to guide clinical practice and standardize wound care for DFUs. Future initiatives should prioritize the integration of such training into community health care systems, fostering collaborative efforts to enhance DFU healing outcomes and ultimately improve patients’ quality of life.
Limitations
The principal limitation of this study is its single-center, single-arm case series design with a relatively small sample size. To confirm the effectiveness of the wound hygiene protocol, future research should involve larger cohorts and longer follow-up periods. Moreover, patients in this study attended the clinic 3 times per week, a frequency that may not be feasible for widespread implementation due to the associated financial and logistical demands. Given the substantial burden of DFUs in the general population, the scalability and cost-effectiveness of this intensive care model warrant careful evaluation.
Conclusion
This case series demonstrates that the implementation of a standardized wound hygiene protocol significantly improves healing outcomes in patients with DFUs. These findings provide valuable evidence for the clinical management of various hard-to-heal wounds and help address the current gap in wound hygiene research among Asian populations. Notably, wound hygiene plays a pivotal role in optimizing the wound healing trajectory. However, while this protocol represents a highly promising and effective therapeutic strategy, its intensive nature—requiring frequent clinical visits and specialized care—may limit its feasibility and scalability in broader health care settings, particularly in resource-limited regions or among large populations of patients with DFU. Moving forward, it is essential to prioritize the development of structured wound hygiene training programs to expand the pool of qualified wound care professionals, strengthen clinical competencies, and explore more accessible models of care delivery. These efforts are crucial to promoting evidence-based wound management and improving patient outcomes on a larger scale.
Author & Publication Information
Authors: Qing Jia, MSN1; Xiaojing Yin, BSN2; Wen Qin, BSN3; and Jiaojiao Bai, BSN, RN1
Acknowledgments: Q.J. and X.Y. contributed equally to this work.
Affiliations: 1Multidisciplinary Diagnostic Clinic for Diabetic Foot, Huadong Hospital Affiliated to Fudan University, Shanghai, China; 2Department of Emergency Medicine, Huadong Hospital Affiliated to Fudan University, Shanghai, China; 3Department of Geriatrics, Huadong Hospital Affiliated to Fudan University, Shanghai, China
Disclosure: The authors disclose no financial or other conflicts of interest.
Ethical Approval: Written informed consent was obtained from all enrolled patients for the publication of potentially identifiable clinical details with accompanying wound images. Ethical approval was obtained from the Ethics Committee of Huadong Hospital, Fudan University (Approval No. 2024K262).
Correspondence: Jiaojiao Bai, BSN, RN; Multidisciplinary Diagnostic Clinic for Diabetic Foot, Huadong Hospital Affiliated to Fudan University, 221 West Yan'an Road, Jing'an District, Shanghai 200040, China; bjj163163@163.com
Manuscript Accepted: May 8, 2025
References
1. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27-32. doi:10.1016/j.jval.2017.07.007
2. Murphy C, Atkin L, Swanson T, et al. Defying hard-to-heal wounds with an early antibiofilm intervention strategy: wound hygiene. J Wound Care. 2020;29(Sup3b):S1-S26. doi:10.12968/jowc.2020.29.Sup3b.S1
3. Gu Y, Diabetic Foot Disease Branch of China International Exchange and Promotive Association for Medical and Health Care (CPAM), Expert Committee on Diabetic Foot Disease of Chinese Chapter of International Union of Angiology (IUA). Chinese guidelines for diagnosis and treatment of diabetic foot. Vasc Investig Ther. 2020;3(2):58–69. doi:10.4103/VIT.VIT_10_20
4. Wagner Jr FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2(2):64-122. doi:10.1177/107110078100200202
5. Murphy C, Atkin L, Vega de Ceniga M, et al. Embedding wound hygiene into a proactive wound healing strategy. J Wound Care. 2022;31(Sup4a):S1-S19. doi:10.12968/jowc.2022.31.Sup4a.S1
6. Murphy C, Mrozikiewicz-Rakowska B, Kuberka I, et al. Implementation of wound hygiene in clinical practice: early use of an antibiofilm strategy promotes positive patient outcomes. J Wound Care. 2022;31(Sup1):S1-S32. doi:10.12968/jowc.2022.31.Sup1.S1
7. Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. doi:10.1016/j.biopha.2019.108615
8. Lazzarini PA, Armstrong DG, Crews RT, et al. Effectiveness of offloading interventions for people with diabetes-related foot ulcers: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2024;40(3):e3650. doi:10.1002/dmrr.3650
















