Fat Pad Augmentation Using Human Adipose Allograft Yields Durable Closure in Recalcitrant Plantar Ulcers
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. Fat pad atrophy or migration leads to high pressure, a key factor in chronic plantar ulcer development. Current standard of care (SOC) only offers temporary pressure relief through off-loading, without addressing underlying fat pad defects. Objective. To assess the intraoperative use of human cryopreserved adipose tissue allograft (hCAT) alongside SOC for patients with chronic recalcitrant plantar ulcers persisting longer than 12 months. Materials and Methods. Eight patients with significant comorbidities, including diabetes mellitus, peripheral vascular disease, and chronic kidney disease, were included in this retrospective case series. The average ulcer size was 3.69 cm² (range, 0.14 cm²-20 cm²) with an average duration of 39.6 months (range, 21 months-59 months). Each patient underwent surgical debridement followed by hCAT implantation in the periwound area, followed by SOC. Outcomes included ulcer closure rate, time to closure, ulcer-free duration, and hCAT-related adverse events. Results. Seven patients (87.5%) achieved complete closure within 2.8 months on average (range, 0.8 months-7.2 months) with a post-closure ulcer-free duration averaging 5.6 months (range, 3.5 months-7.7 months) at the time of data analysis. No hCAT-related adverse events were observed. Patients are still being followed for longer-term outcomes. Conclusion. These findings support hCAT as an effective adjunct to SOC in the management of chronic recalcitrant plantar ulcers.
Introduction
The fat pad on the plantar surface of the foot functions as a crucial cushion, absorbing shock and protecting underlying structures during weight-bearing activities. When the fat pad degenerates or displaces, plantar pressure on bony prominences increases, leading to skin breakdown and subsequent ulcer formation.1 Without effective intervention, these ulcers often become chronic and recurrent.
Chronic recalcitrant plantar ulcers are those that do not respond to treatment over time, typically showing minimal or no improvement after 4 or more weeks of care.2 Factors contributing to the challenging nature of these ulcers include underlying health conditions such as diabetes, obesity, and cardiovascular disease.3 The extended duration of plantar ulcers increases their vulnerability to infection, which can elevate amputation risk to as high as 80%.4 This escalation in risk contributes to increased morbidity and mortality rates, collectively imposing a significant economic burden on health care systems.5
While advanced therapies can support ulcer healing, they do not address the underlying fat pad deficiency that contributes to elevated plantar pressure. Off-loading devices incorporated as part of standard of care (SOC) provide only temporary relief, while more invasive surgical corrections such as osteotomies and tendon balancing can reduce plantar pressure more permanently; neither approach addresses the underlying fat pad deficiency, leaving patients at high risk for recurrence.6 Consequently, reconstructing the adipose layer beneath the foot remains an essential yet unmet need in managing chronic plantar ulcers.
A recently developed human cryopreserved adipose tissue allograft (hCAT) (Liposana; Britecyte, Inc) presents a novel solution for conditions associated with adipose deficiencies. hCAT is available on demand and can be used for any patient without requiring matching or immunosuppression. The processing of hCAT preserves the natural structure and cushioning properties of native adipose tissue, making it particularly beneficial for conditions characterized by adipose deficiency and compromised cushioning, such as plantar ulcers.
In this retrospective case series, hCAT was utilized as an adjunct to SOC to address fat pad deficiencies in patients with chronic recalcitrant plantar ulcers.
Materials and Methods
hCAT is manufactured by LifeLink Foundation, Inc, and is classified as a human cells, tissues, and cellular- and tissue-based product under Section 361 of the Public Health Service Act and Title 21, CFR Part 1271. It is sourced and processed aseptically from rigorously screened donors, adhering to the standards set by the American Association of Tissue Banks and all relevant state and federal regulations.7
Intended for the repair, replacement, or reconstruction of adipose tissue defects, hCAT maintains a shelf life of up to 5 years when stored at −40°C or lower. If stored between −20°C and −40°C, its use is limited to 6 months.
The product is provided in a sterile 2-mL cryogenic vial with a screw cap, containing 1.5 mL of hCAT. It is sealed within dual chevron-style peel pouches and shipped on dry ice. To preserve integrity, the product must remain at the recommended temperature until thawed, after which it should be used the same day.
This retrospective case series evaluated the clinical outcomes of hCAT in patients with chronic recalcitrant plantar ulcers. Patients in this study were enrolled at a nonacademic private suburban podiatry practice. To be eligible for hCAT application, patients were required to have a chronic plantar ulcer of at least 12 months in duration, be at least 18 years old, and have not previously achieved closure with SOC and at least 1 advanced therapy. Furthermore, ulcer drainage needed to be minimal with no active infection present. Patients were also selected based on their history of adherence with follow-up visits and their motivation to actively manage their ulcers. Due to the chronic nature of the ulcer and the presence of necrotizing and nonviable tissue, patients underwent surgical debridement in a hospital-based outpatient setting.
All patients provided written consent prior to implantation. Data were collected from patient medical records and were de-identified in compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA). The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. Because this was a retrospective analysis, institutional review board approval was waived.
Product preparation and application
In the operating room (OR), patients were placed under general anesthesia. The ulcer bed was cleansed with betadine using standard sterile technique. The ulcer was then debrided with a No. 15 blade scalpel, ensuring clean ulcer edges. In cases where there was significant scar tissue adhering to underlying structures, a tissue plane was created either through the ulcer bed or via a small stab incision. Pre-debridement and post-debridement measurements were taken intraoperatively.
To prepare hCAT for implantation, the outer pouch was discarded, keeping the vial enclosed in the inner pouch, which was then positioned on a sterile surface in the OR. The product was allowed to thaw for about 30 minutes. After thawing, the vial was removed from the pouch and gently agitated, and its contents were promptly drawn into a syringe fitted with an 18-gauge needle.
hCAT was implanted subcutaneously around the ulcer using a so-called donut technique, in which the product was placed at approximately 4 to 5 sites circumferentially with the greatest soft tissue deficit in the peri-ulcer region, typically 0.5 cm from the ulcer margin. Depending on the size of the ulcer, patients received either 1.5 mL or 3.0 mL of hCAT. After application, the implant sites were sealed with a high-viscosity, 2-octyl cyanoacrylate topical skin adhesive (exofin; Chemence Medical). The ulcer was then covered with gauze dressing (Xeroform; Covidien), gauze pads (ABD; McKesson) if appropriate, and bandage rolls (Kerlix; Covidien).
Postoperative care and monitoring
Off-loading instructions were tailored to each patient. Off-loading methods included foam padding, shoe inserts, custom orthoses, and/or an off-loading boot. Postoperative visits occurred within 1 week. Patients were initially followed up weekly, with visit frequency adjusted based on ulcer closure progress. Dressings were changed at weekly visits or at home in between appointments. Throughout follow-up, ulcers were cleaned and debrided, and patients maintained off-loading of the ulcer site.
Outcome measures
Clinical outcomes included complete ulcer closure, time to closure, ulcer-free time, and recurrence. Complete ulcer closure was defined as 100% reepithelialization. Additional outcomes included percentage area reduction for non-closed ulcers and hCAT-related adverse events.
Results
Between November 2023 and May 2024, 8 patients with chronic plantar ulcers received a single intraoperative hCAT implant. Patient demographics, ulcer characteristics, and outcomes are summarized in Table 1. Of the 8 patients who received hCAT, 5 were male and 3 were female, with an average age of 61.1 years (range, 45 years-80 years).
Seven patients (87.5%) had diabetes mellitus (DM), 6 (75%) had a history of hypertension, 6 (75%) had a history of hyperlipidemia, and 5 (62.5%) had peripheral vascular disease (PVD). PVD was defined in this study using objective hemodynamic and imaging criteria. Patients with ankle-brachial index (ABI) values less than or equal to 0.90 were classified as having peripheral artery disease, consistent with established diagnostic thresholds. In individuals with end-stage renal disease (ESRD) and DM—in whom arterial calcification may reduce the reliability of ABI measurements—additional evaluation was performed using the toe brachial index, with values less than 0.70 considered indicative of distal arterial disease. Duplex Doppler ultrasonography and computed tomography angiography of the lower extremities were used to further characterize arterial pathology, particularly in cases of clinically complex or multilevel disease. Hemodynamically significant stenosis was defined as greater than or equal to 50% luminal narrowing on cross-sectional or contrast-enhanced vascular imaging. Most patients underwent either endovascular or open surgical revascularization prior to graft intervention, with at least single-vessel distal runoff to the affected extremity to support graft patency and optimize limb salvage outcomes. Additional medical histories included chronic kidney disease, obesity, gastroesophageal reflux disease, congestive heart failure, anemia, and ESRD. The average ulcer size was 3.69 cm2, with an average duration of 39.6 months.
Seven of the 8 patients who received hCAT achieved complete ulcer closure (87.5%), with an average time to closure of 2.8 months. Five of these ulcers achieved closure by 12 weeks. Among the 7 patients who achieved complete closure, 2 experienced a recurrence. One patient’s ulcer reopened after 5.6 months due to nonadherence, leading to repeated injury to the area. Another patient experienced reopening 1.1 months after closure due to a foot injury, but successfully achieved closure again within 1 month without additional hCAT application. The patient who did not achieve full closure showed a 93.8% reduction in ulcer area at 6 months. As of this writing, the 5 patients with closed ulcers have remained ulcer-free for an average of 5.6 months (range, 3.5 months–7.7 months), with ongoing monitoring for long-term outcomes. Ulcers that remained closed for at least 3 months were classified as having achieved durable closure, a milestone reached by all closed ulcers in the study.
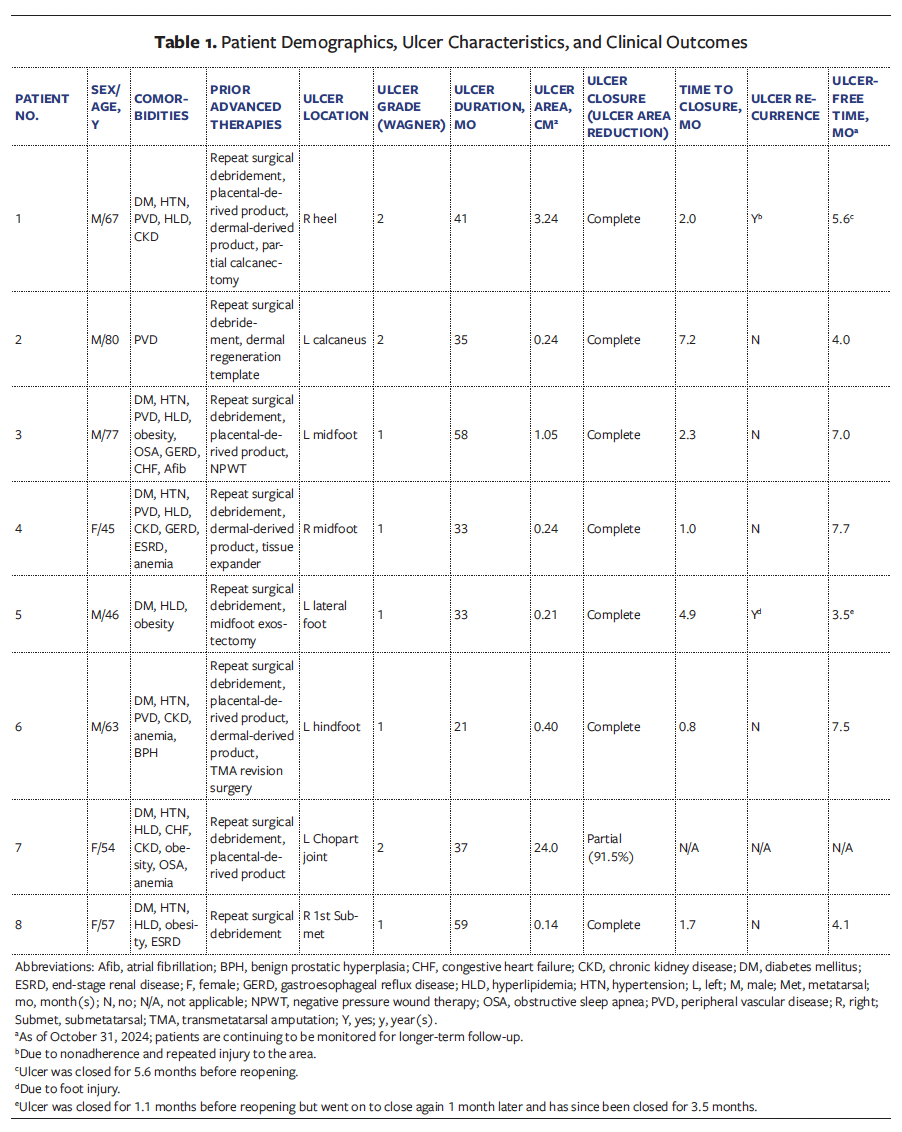
No product-related adverse events were reported. Photographs of wound progression from a representative patient (Case 4) are shown in the Figure.
Discussion
Chronic recalcitrant ulcers on the plantar surface of the foot present a significant clinical challenge, primarily due to the limited treatment options available for addressing fat pad deficiency, which leads to elevated plantar pressure—a key factor in ulcer formation and recurrence.1-3 The inability of standard treatments to remedy fat pad deficiency highlights an unmet medical need in plantar ulcer management. To address this unmet medical need, hCAT has been developed as a novel solution for the repair, replacement, or reconstruction of adipose tissue defects. The present retrospective case series evaluated the application of hCAT alongside SOC in patients with chronic plantar ulcers.
Regulski et al8 previously investigated the application of hCAT in a patient with diabetic neuropathy and a history of recurrent plantar ulcers. The patient presented with a pre-ulcerative lesion on the left heel and received an hCAT implant. Two weeks postimplantation, the patient transitioned from an off-loading boot to a custom brace and diabetic shoe. At 9-month follow-up, no new ulcers had formed, the hCAT implant remained palpable, the callus had resolved, and no adverse events were reported.8
Although the case reported by Regulski et al8 did not involve an open ulcer, reported outcomes led to the hypothesis that hCAT provides structural support to areas with fat pad deficiency that protects these areas from plantar ulcer formation and recurrence. To test this hypothesis, patients with chronic plantar ulcers requiring surgical debridement due to chronicity and the presence of necrotic tissue were selected for intraoperative hCAT implantation for the current case series. All patients had ulcers that persisted for at least 12 months and were unresponsive to both SOC and advanced therapies.
In this study cohort, the average ulcer size was 3.69 cm², with an average duration of 39.6 months. Of the 8 patients, 7 (87.5%) achieved complete ulcer closure with an average time to closure of 2.8 months. Clinical outcomes observed in this study were compared with clinical outcomes for chronic plantar ulcers reported in the literature (Table 2).9-17
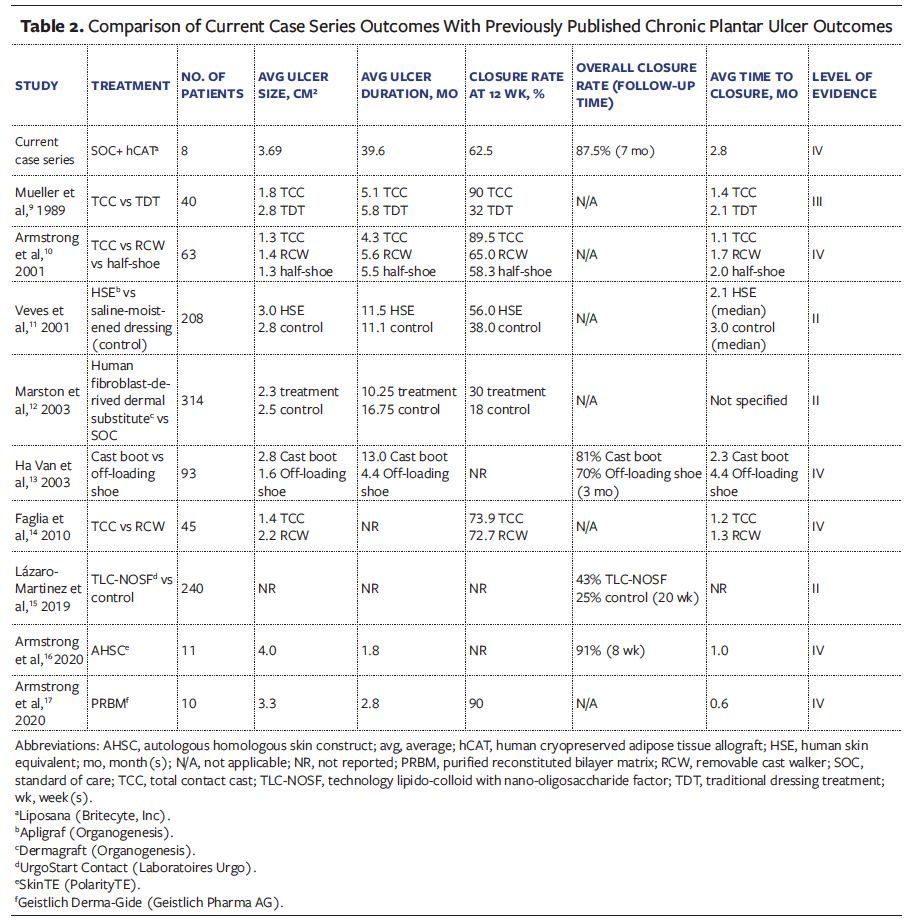
In contrast to this study, published literature generally reports outcomes for smaller ulcers with significantly shorter durations (Table 2).9-17 Ulcer size and duration are major predictive factors for ulcer closure, with larger, more chronic ulcers often exhibiting lower closure rates.18 Limited data on ulcers older than 11 months report closure rates of only 19% to 30%.15,19 Achieving an 87.5% closure rate in the current study, in which nearly all ulcers persisted beyond 2 years, highlights hCAT’s effectiveness in highly chronic, recalcitrant plantar ulcers. Furthermore, ulcer-free duration data were not reported in the majority of literature studies. While positive closure outcomes were reported, it remains challenging to assess the long-term durability of ulcer closures.
Although current treatments may promote reepithelialization, newly closed ulcers often develop unstable scars with thin skin adhered to underlying structures, making them vulnerable to re-
ulceration. Recurrence rates are reportedly as high as 50% within 2 months post-closure.20 In the current study, 2 of the 7 patients (28.6%) experienced re-ulceration: 1 occurred nearly 6 months post-closure following a foot injury, while the other reopened but closed within 1 month and remained closed thereafter. Participants of this study remained ulcer-free for an average of 5.6 months (range, 3.5 months-7.7 months) with extended follow-up ongoing, suggesting that implanted hCAT may provide long-lasting protection to ulcer-prone areas.
For plantar fat pad atrophy and displacement specifically, treatment options are incredibly limited. In early studies, Balkin21 evaluated 32 plantar ulcer sites (29 post-healing and 3 pre-closure) treated with silicone, finding that 23 of the healed sites showed no recurrence over 13- to 201-month follow-up, while those injected pre-healing showed minimal improvement over 2 years. Similarly, van Schie et al22 demonstrated that liquid silicone injections increased plantar thickness and reduced pressure for up to 12 months in patients with diabetic neuropathy, potentially aiding in ulcer prevention in high-risk feet. However, data on open ulcers treated with silicone remain limited, and concerns regarding its long-term safety restrict its broader use.21,22
More recent studies have examined autologous fat grafting for cases of fat pad atrophy. In a 2016 case report, Luu et al23 detailed a 37-year-old patient with diabetes, with a history of gangrene and residual fat pad atrophy, who was able to resume regular footwear without ulcer recurrence after undergoing fat grafting.
Kress et al24 retrospectively reviewed 15 neuropathic patients with 17 pedal ulcers who received either autologous fat grafting or an adipose allograft matrix (Leneva; MTF Biologics) on the plantar surface. Among these, 7 patients with DM had ulcers with an average size of 1.24 cm2. All ulcers closed in an average of 1 month. One patient with spina bifida had an ulcer measuring 0.5 cm2 and received the adipose allograft matrix, but time to closure was not reported. Additionally, 1 patient with Parkinson’s disease had a lesion measuring 1.08 cm2 and received the adipose allograft matrix, which closed within 14 days. During an average follow-up period of 6.9 months, both groups demonstrated positive outcomes, with no complications or ulcer recurrence, highlighting the potential benefits of these therapies for ulcer prevention.24
While autologous grafting has shown promising results, its limitations include the risk of donor site morbidity and an unpredictable adipose graft resorption rate, which can lead to inconsistent clinical outcomes.25-27
Limitations
Limitations of the current study include its retrospective design, small sample size, and absence of a control group. The small sample size and retrospective design also limit conclusions regarding the cost-effectiveness of hCAT. Although adipose allograft matrix offers a potential alternative to autologous grafting, the limited data on its use in open ulcers makes its effectiveness in this application uncertain. Larger, randomized controlled trials are warranted to further investigate the findings of the current study, and studies on the cost-effectiveness of hCAT are needed.
Conclusion
This case series represents the first report of intraoperative adipose tissue allograft use for addressing fat pad defects in chronic recalcitrant plantar ulcers. Positive clinical outcomes noted in this study indicate that hCAT should be considered by clinicians as an adjunct to their current protocols to manage chronic recalcitrant plantar ulcers. The favorable outcomes in patients with multiple comorbidities and recalcitrant plantar ulcers of over 12-month chronicity warrant larger, randomized controlled trials. The small sample size and retrospective design also limit conclusions regarding the cost-effectiveness of hCAT, which will need to be addressed in future studies.
Author & Publication Information
Authors: Kristen McGinness, DPM; Brad Peck, DPM; Usman Javed, DPM; Amer Hitto, DPM; Torrin Lundberg, DPM; and Devin Ricks, DPM
Affiliation: Cape Fear Valley Podiatry, Fayetteville, NC, USA
Acknowledgments: Molly Saunders, Senior Director, Regenerative Medicine Programs. Britecyte, Inc, assisted with drafting and reviewing the manuscript. Alla Danilkovitch, PhD, Founder and Chief Scientific Officer of Britecyte, also assisted with reviewing the manuscript.
Disclosure: The authors disclose no financial or other conflicts of interest.
Ethical Approval: All patients provided written consent prior to implantation. Data were de-identified in compliance with HIPAA (Health Insurance Portability and Accountability Act of 1996), and the study was conducted in accordance with the Declaration of Helsinki. Given the retrospective nature, institutional review board approval was waived.
Correspondence: Kristen McGinness; Cape Fear Valley Podiatry, 1645 Owen Dr, Fayetteville, NC 28304; KMcginness@capefearvalley.com
Manuscript Accepted: July 2, 2025
References
pad and the diabetic foot—a review. Int Wound J. 2015;12(6):636-640. doi:10.1111/iwj.12173
2. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763-771. doi:10.1111/j.1524-475X.2009.00543.x
3. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle). 2015;4(9):560-582. doi:10.1089/wound.2015.0635
4. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13(5):513-521. doi:10.2337/diacare.13.5.513
5. Sen CK. Human wound and its burden: updated 2022 compendium of estimates. Adv Wound Care (New Rochelle). 2023;12(12):657-670. doi:10.1089/wound.2023.0150
6. Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. J Vasc Surg. 2010;52(3 Suppl):37S-43S. doi:10.1016/j.jvs.2010.06.007
7. Liposana Package Insert. Britecyte, Inc.; 2024.
8. Regulski MJ, Saunders MC, McCulloch SE. Adipose tissue allograft for the management of a pre-ulcerative plantar lesion in a diabetic neuropathic patient. Clin Case Rep. 2024;12(9):e9330. doi:10.1002/ccr3.9330
9. Mueller MJ, Diamond JE, Sinacore DR, et al. Total contact casting in treatment of diabetic plantar ulcers. Controlled clinical trial. Diabetes Care. 1989;12(6):384-388. doi:10.2337/diacare.12.6.384
10. Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off-loading the diabetic foot wound: a randomized clinical trial [published correction appears in Diabetes Care. 2001;24(8):1509]. Diabetes Care. 2001;24(6):1019-1022. doi:10.2337/diacare.24.6.1019
11. Veves A, Falanga V, Armstrong DG, Sabolinski
ML; Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24(2):290-295. doi:10.2337/diacare.24.2.290
12. Marston WA, Hanft J, Norwood P, Pollak R; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26(6):1701-1705. doi:10.2337/diacare.26.6.1701
13. Ha Van G, Siney H, Hartmann-Heurtier A, Jacqueminet S, Greau F, Grimaldi A. Nonremovable, windowed, fiberglass cast boot in the treatment of diabetic plantar ulcers: efficacy, safety, and compliance. Diabetes Care. 2003;26(10):2848-2852. doi:10.2337/diacare.26.10.2848
14. Faglia E, Caravaggi C, Clerici G, et al. Effectiveness of removable walker cast versus nonremovable fiberglass off-bearing cast in the healing of diabetic plantar foot ulcer: a randomized controlled trial. Diabetes Care. 2010;33(7):1419–1423. doi:10.2337/dc09-1708
15. Lázaro-Martínez JL, Edmonds M, Rayman G, et al. Optimal wound closure of diabetic foot ulcers with early initiation of TLC-NOSF treatment: post-hoc analysis of Explorer. J Wound Care. 2019;28(6):358-367. doi:10.12968/jowc.2019.28.6.358
16. Armstrong DG, Orgill DP, Galiano R, et al. Complete wound closure following a single topical application of a novel autologous homologous skin construct: first evaluation in an open-label, single-arm feasibility study in diabetic foot ulcers. Int Wound J. 2020;17(5):1366-1375. doi:10.1111/iwj.13404
17. Armstrong DG, Orgill DP, Galiano RD, et al. An observational pilot study using a purified reconstituted bilayer matrix to treat non-healing diabetic foot ulcers. Int Wound J. 2020;17(4):966-973. doi:10.1111/iwj.13353
18. Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: the association of wound size, wound duration, and wound grade on healing. Diabetes Care. 2002;25(10):1835-1839. doi:10.2337/diacare.25.10.1835
19. Ananian CE, Davis RD, Johnson EL, et al. Wound closure outcomes suggest clinical equivalency between lyopreserved and cryopreserved placental membranes containing viable cells. Adv Wound Care (New Rochelle). 2019;8(11):546-554. doi:10.1089/wound.2019.1028
20. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367-2375. doi:10.1056/NEJMra1615439
21. Balkin SW. Injectable silicone and the foot: a 41-year clinical and histologic history. Dermatol Surg. 2005;31(11 Pt 2):1555-1560. doi:10.2310/6350.2005.31241
22. van Schie CH, Whalley A, Vileikyte L, Wignall T, Hollis S, Boulton AJ. Efficacy of injected liquid silicone in the diabetic foot to reduce risk factors for ulceration: a randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23(5):634-638. doi:10.2337/diacare.23.5.634
23. Luu CA, Larson E, Rankin TM, Pappalardo JL,
Slepian MJ, Armstrong DG. Plantar fat grafting and tendon balancing for the diabetic foot ulcer in remission. Plast Reconstr Surg Glob Open. 2016;4(7):e810. doi:10.1097/GOX.0000000000000813
24. Kress GT, Swerdlow M, Mohan N, Patel K, Shin L. Remission strategies with fat grafting to prevent recurrence of pedal ulcerations and pain: a case series. Plast Reconstr Surg Glob Open. 2023;11(9):e5232. doi:10.1097/GOX.0000000000005232
25. Wederfoort JLM, Hebels SA, Heuts EM, van der Hulst RRWJ, Piatkowski AA. Donor site complications and satisfaction in autologous fat grafting for breast reconstruction: a systematic review. J Plast Reconstr Aesthet Surg. 2022;75(4):1316-1327. doi:10.1016/j.bjps.2022.01.029
26. Rohrich RJ, Sorokin ES, Brown SA. In search of improved fat transfer viability: a quantitative analysis of the role of centrifugation and harvest site. Plast Reconstr Surg. 2004;113(1):391-397. doi:10.1097/01.PRS.0000097293.56504.00
27. Doornaert M, Colle J, De Maere E, Declercq H, Blondeel P. Autologous fat grafting: latest insights. Ann Med Surg (Lond). 2018;37:47-53. doi:10.1016/j.amsu.2018.10.016
















