A Combined Multistep Reconstruction of the Heel After Skin Tumor Resection in Posttraumatic Chronic Ulcers: A Case Series
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. Heel reconstruction following wide resection of Marjolin ulcer represents a challenge for surgeons. Local or free flaps are usually used, but they present some disadvantages, such as donor site morbidity, high risk of necrosis, and long surgical time and hospital stay. Objective. To use a combined multistep protocol of heel reconstruction after wide resection of Marjolin ulcer using a biosynthetic extracellular matrix and negative wound pressure therapy. Materials and Methods. Demographic, clinical, and surgical data for 4 patients treated at a single institution were retrospectively collected. Results. All patients healed with a good scar appearance and high satisfaction. No complications or tumor recurrence were observed at 12 months postoperative. Conclusion. The findings of this clinical series suggest that the combined protocol described is an effective option for the resolution of these complex wounds.
Plastic surgeons may encounter challenges when reconstructing the heel following tumor removal in patients with posttraumatic chronic ulcers, or Marjolin ulcer (MU). MU is a rare, aggressive ulcerating cancer that develops in damaged or chronically inflamed skin or scarred tissues. It has a low incidence rate and an unclear cause.1 Chronic inflammation likely results in mutations in focal cells. Biopsies are used to diagnose MU, and wide resection is required. When MU develops in the heel, the repair is complex.1 The serious clinical difficulties that need to be resolved are the site and extent of resection, the risk of tumor recurrence, inadequate vascular flow, and comorbidities. An ideal reconstruction would replace lost tissue in “like by like” fashion while preserving function, would cover exposed planes (bones or fascia) with soft tissue bulk, and would produce aesthetically acceptable results. Although there are advantages to using local or free flaps, there are also disadvantages, such as donor site morbidity, a high risk of necrosis in the localized reduced vascular network, longer hospital stays, and long surgical time. As a result, tissue-engineered dressings have greater application potential than flap surgery.
Dermal substitutes are a promising method for treating soft tissue abnormalities and improving wound healing quality. To minimize immunogenicity, these biological grafts have no cellular or antigenic components. These products are derived from human, bovine, and porcine tissues and contain collagen fibers, fibronectin, elastin, laminin, glycosaminoglycans, and hyaluronic acid. They act as a framework that is gradually vascularized and cellularized by the host. Because of the absence of an epidermis, the dermal substitute is not intended to replace a whole layer of skin tissue. It replaces the necessity for a full-thickness skin graft with a thin layer of skin graft, resulting in minimal scar formation at the donor site.2
Thus, the dermal substitute may provide a viable, simple, and reliable alternative therapeutic technique that can yield long-term functional and cosmetic results with a minimal incidence of complications. Only a few reports on the management of these complex reconstructions have been published to date.
The present report discusses a clinical series of 4 consecutive patients who underwent effective multistep repair after extensive excision of squamous cell carcinoma (SCC) of the heel (in chronic posttraumatic ulcers, MU). In particular, the biosynthetic extracellular matrix (BECM) Pelnac (Gunze Ltd; hereafter “proprietary BECM”) was used. This collagen-based wound dressing consists of 2 layers: a porcine tendon-derived atelocollagen sponge layer, which provides a scaffold for cellular invasion and capillary growth (dermal regeneration), and an overlying semipermeable silicone film layer that acts as a temporary epidermis. When the dermis-like tissue formation is complete, as signaled by the appearance of a red granulation-like tissue, the outer silicone layer can be easily removed, and coverage with a thin split-thickness skin graft (STSG) can be achieved.
Materials and Methods
Patients
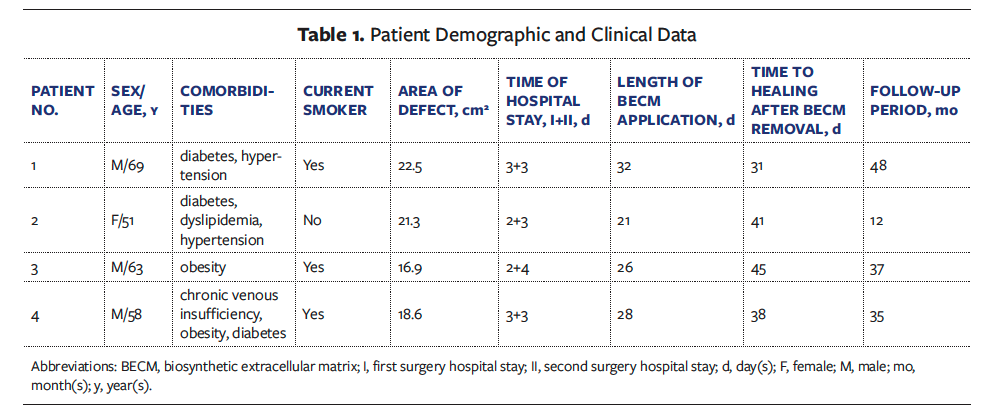
A retrospective evaluation of 4 consecutive patients examined in the Plastic Surgery Department of Policlinico Riuniti – University of Foggia, a tertiary referral hospital in Southern Italy, between January 2019 and January 2022 was conducted. Broad excision of SCC of the heel in posttraumatic ulcers was performed, followed by rebuilding with the proprietary BECM. Patients were evaluated based on demographic and clinical data (Table 1). All patients gave written informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
Surgical technique
Step by step, the management strategy was as follows: incisional biopsies for diagnosis, wide excision of neoplasm according to the Italian Association of Medical Oncologists (AIOM) guidelines for cutaneous squamous cell carcinoma, coverage with a dermal substitute, application of negative pressure wound therapy (NPWT), final coverage with STSG, and new application of NPWT with a nonadherent silicone dressing interface (Mepitel One; Mölnlycke).
In particular, after biopsy revealed SCC, the patients underwent 2 surgeries under locoregional anesthesia and, if needed, sedation. Every patient received short-term antibiotic prophylaxis consisting of 2 g of cefazolin. According to the AIOM guidelines, the first step was to remove the tumor. Fresh frozen sections were not available. For SCC that had spread to bone, osteotomy was required to remove the tumor and access the bleeding spots. The defect was corrected utilizing a combination of the new bilayer BECM and an NPWT device (3M V.A.C.; Solventum). The dermal substitute was stapled in place, and an NPWT device set to −125 mm Hg was attached directly to the dermal matrix’s fenestrated silicone layer (Figure 1) until the first dressing.
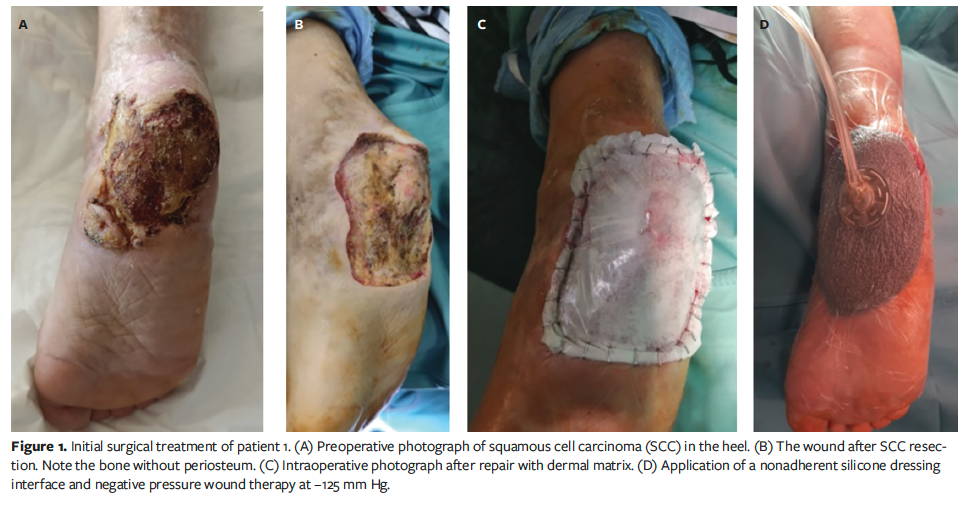
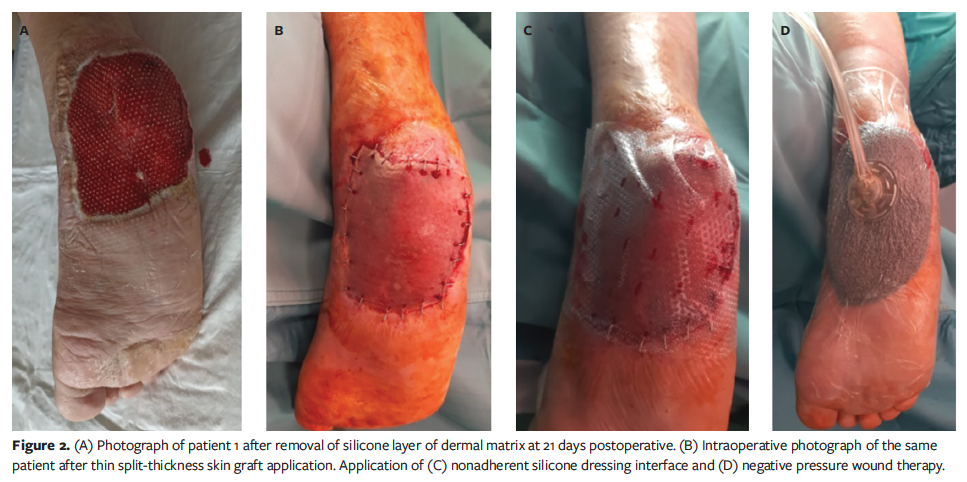
The first dressing change occurred on postoperative days 5 to 7. The wound bed was examined once a week to assess the adherence of the silicone layer and the vascularization status (coloration of tissues beneath the transparent silicone layer), and to identify any complications, such as hematoma or infection. Typically, the second treatment was performed 3 weeks later, after complete revascularization of the proprietary BECM was achieved and after the final histological analysis confirmed tumor-free margins. The second treatment involved removing the surface silicone layer and covering it with an STSG from the thigh, followed by the aforementioned nonadherent silicone dressing interface underneath the NPWT device set at −125 mm Hg (Figure 2). At 7 days postoperative, NPWT was removed, the wound was evaluated for healing, and dressings were used as needed until healing was complete (Figure 3).
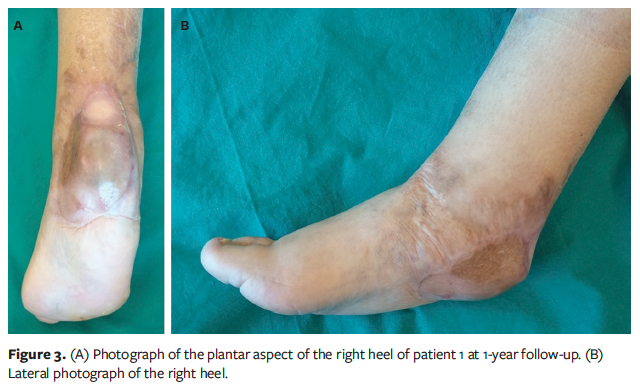
At 12-month follow-up, the authors assessed tumor recurrence, scar quality (using the Vancouver Scar Scale [VSS]),3 and patients’ health-related quality of life using the 17-item Wound-QoL-17 questionnaire.4 Each item of the Wound-QoL-17 is assessed on a 5-point Likert scale, with 0 representing “not at all” and 4 representing “very much.” The global score is calculated by averaging the ratings for all questions, ranging from 0 (the best score) to 68 (the worst).
Results
A total of 4 patients’ medical records were reviewed (Table 1). Three patients (75%) were male, and 1 was female (25%). The mean (standard deviation [SD]) patient age was 60.25 (6.61) years (median, 60.5 years; range, 51 years-69 years). The follow-up period lasted from 12 months to 48 months (mean [SD], 33 [13.09] months; median, 36 months). No adjuvant radiation or sentinel lymph node biopsy was carried out. The mean (SD) surface area defect after tumor excision was 19.82 (2.20) cm2 (median, 19.95 cm2; range, 16.9 cm2-22.5 cm2). In 2 cases (50%), the surgical bed was bone with no periosteum. There were no serious issues after the first operation.
There were no issues with the artificial dermal matrix application. When a well-vascularized neodermis was clinically detected, STSG was performed; this occurred approximately 21 days to 30 days after administration of the proprietary BECM (mean [SD] 26.75 [3.96] days; range, 21 days-32 days; median, 27 days). The average (SD) length of hospital stay was 2.5 (0.5) days after the initial surgery (range, 2 days-3 days; median, 2.5 days) and 3.25 (0.43) days (range, 3 days-4 days; median, 3 days) after the second surgery.
All patients had 100% graft take. The average (SD) healing period was 38.75 (5.12) days (median, 39.5 days; range, 31 days-45 days).
At 1 year after surgery, no tumor recurrence was found. The scar appearance was evaluated using the VSS by an expert plastic surgeon who was masked to patient identities. The mean VSS score was 4.75, indicating that all patients achieved considerable acceptable aesthetic results. At the same time, the patients’ quality of life was assessed using the Wound-QoL-17 questionnaire. The results were compared with preoperative values and revealed a patient-relevant change in all items of the 3 subscales: everyday life, body, and psyche. The average (SD) score was 46.25 (4.44) (median, 47.5; range, 39-51) preoperative and 12.25 (3.11) (median, 11.5; range, 9-17) postoperative (Table 2).
Case 1
A 69-year-old male developed SCC in a chronic heel ulcer as a result of mechanical trauma. He had a history of diabetes, hypertension, and smoking. After punch biopsy revealed the presence of SCC, wide excision of the tumor was performed (as per the guidelines), and the proprietary BECM was applied to the incision using NPWT. After 32 days, a second surgery was performed to remove the silicone layer and finish the restoration with a skin graft, with NPWT for another week. After an additional 31 days of follow-up, complete wound healing was achieved.
Case 2
A 51-year-old female with a history of diabetes, dyslipidemia, and hypertension developed an SCC in a chronic heel lesion caused by burn. Following diagnostic biopsies, the patient underwent SCC excision and initial repair using the dermal substitute graft and NPWT. After 21 days, a skin graft was performed, followed by 7 days of NPWT. Complete wound healing was observed after 41 days of follow-up after BECM removal.
Case 3
A 63-year-old male developed SCC in a chronic heel lesion caused by post-
thrombotic syndrome. He smoked heavily (>40 cigarettes/day) and had obesity. Following a positive biopsy for SCC and subsequent resection, the surgical protocol was implemented. After 26 days of treatment with the proprietary BECM, skin grafting was performed and NPWT started. After an additional 45 days, the wound was completely healed.
Case 4
A 58-year-old male developed an SCC in a traumatic heel ulcer. Comorbidities included chronic venous insufficiency, obesity, and diabetes, in addition to heavy smoking (>40 cigarettes/day). Wide excision of the SCC was performed, followed by rebuilding using the proprietary BECM and NPWT. After 28 days, the second phase of reconstruction using a skin graft and 7 days of NPWT was completed. Complete wound closure was achieved at 38-day follow-up after BECM removal.
Discussion
The anatomical and functional qualities of the heel make it challenging to recreate. Following broad excision of a malignant skin cancer in posttraumatic wounds, reconstruction offers several challenges, including a loss of skin vascularization and mobility, the need for full-thickness restoration, and function maintenance (stability and walking). There are multiple treatment approaches available to manage various types of wounds, including skin graft, NPWT, bioengineered tissue substitutes, pedicle flaps, and free tissue transfer. Treatments are organized hierarchically based on the so-called reconstruction pyramid, which serves as a wound healing guide, ranging from minimally invasive operations for tiny and superficial wounds to extremely sophisticated procedures for large, deep, and complicated soft tissue defects. Following the basic reconstructive principle of replacing like by like is challenging due to the distinct microanatomy and structure of the foot’s plantar surface as well as the scarcity of donor sites.5
Therefore, it is difficult to fully restore the functionality of the plantar foot after trauma, including its ability to support weight and compression during walking. STSGs, like the pinch graft, still play a role in foot reconstruction. Pinch and punch grafts are specific examples of island grafts (ie, seed grafts), which are small pieces of full-thickness skin graft or STSG that are placed into a granulating wound bed. These techniques are technically easy to perform, and several of the new surgical dressings can be combined to shorten the time for the development of granulation tissue within a clean ulcer base and improve the survival of the grafts themselves.5
Additionally, using cultured epithelial autograft procedures, massive full-
thickness damage to the sole of the foot can be covered. Large epithelial sheets can be produced from comparatively small tissue samples using this technique, which is a helpful substitute when donor sites are insufficient, defects are significant, multiple traumas are present, or the patient is not a good candidate for lengthy, invasive surgery. Flap coverage is required for any exposed arteries, tendons, or bone. Many reconstructive flaps have been reported; however, they have several drawbacks, including secondary injury, poor tissue appearance, and significant surgical requirements, or they are not suitable for local or systemic disorders. As a result, tissue-engineered dressings have greater application potential than flap surgery. Acellular dermal matrix (ADM) and BECM are promising methods for treating soft tissue abnormalities and improving wound healing quality. Beginning approximately 3 decades ago, tissue-engineered dressings have been used to repair deep tissue abnormalities alongside STSGs and NPWT. Because of the absence of an epidermis, they are not intended to replace a whole layer of skin tissue. ADM and BECM replace the necessity for a full-thickness skin graft with a thin layer of skin graft, resulting in minimal scar formation at the donor site.5-19 Thus, heel reconstruction can be accomplished in multiple steps utilizing an ADM or a BECM and NPWT. This combination is beneficial because negative pressure promotes adhesion of the artificial dermis to the uneven wound surface, eliminates exudates, maintains a clean wound environment, and stimulates the underlying wound bed.
The present case series shows that the proprietary BECM utilized is effective and safe, and it may be a viable alternative to local or free flaps. Suzuki et al20 initially defined the artificial dermis (proprietary BECM) as an acellular double-layer synthetic device composed of an exterior silicone (polysiloxane) semipermeable membrane and an inner three-dimensional porous collagen matrix. The outer layer defends against mechanical and infectious damage, regulates temperature and humidity, and inhibits hypergranulation. The inner layer is an acellular dermal scaffold composed of atelocollagen, which promotes cellular ingrowth. The dermal matrix collagen produces a low antigenic response, and it is eventually replaced by endogenous collagen, resulting in a new elastic, consistent, and collagenase-resistant dermal layer. After approximately 21 days, the outer layer can be removed and the neodermis covered with an autologous thin STSG.20-26
The main reasons for using BECM in patients with heel wounds following wide resection of MU are that it provides durable coverage, is thicker than a skin graft, has associated minor complications compared with local or free flap, affords early detection of recurrence, and provides instantaneous temporary wound closure protection, thereby preventing infection and moisture loss, until the final pathologic examination results are known.20-26
Limitations
The use of the BECM has many advantages, including fast availability, low donor site morbidity, the capacity to fill huge abnormalities, good cosmetic results, and quick recovery. The main disadvantages are the need for a second treatment and the device’s cost. 21-23 Limitations of the present study are the small sample size and the involvement of only one anatomic site.
Conclusion
In the clinical cases presented in this report, combined multistep reconstruction of the heel after skin tumor resection in posttraumatic chronic ulcer using BECM and NPWT delivered tissue of appropriate thickness to fill a large heel deformity with good long-term aesthetic and functional outcomes, no side effects, and high patient satisfaction (as evidenced by Wound-QoL-17 questionnaire answers). Although these results are preliminary and the sample size is small, they indicate that the combination of BECM and NPWT is an effective, easy, safe, and legitimate treatment alternative for the resolution of these challenging wounds. To the authors’ knowledge, this is the first report in the literature on the use of that combined treatment approach for heel wounds. More research is needed to enhance the sample size and compare it with other BECMs and/or ADMs.
Author and Public Information
Authors: Fedele Lembo, MD; Liberato Roberto Cecchino, MD; Domenico Parisi, MD; and Aurelio Portincasa, MD
Affiliation: Unit of Reconstructive and Plastic Surgery, Ospedali Riuniti di Foggia, Foggia, Italy
Disclosure: The authors disclose no financial or other conflicts of interest.
Ethical Approval: This study was conducted in compliance with the current Good Clinical Practice standards and in accordance with the relevant guidelines and regulations and the principles set forth in the Declaration of Helsinki. All participants in the study gave written informed consent.
Correspondence: Lembo Fedele; Unit of Reconstructive and Plastic Surgery, Policlinico Ospedali Riuniti di Foggia, Foggia 71122, Italy; lembofedele@gmail.com
Manuscript Accepted: March 27, 2025
References
1. Mitra S, Panda S, Sikka K, Mallick S, Thakar A. Multimodality management of locoregionally extensive Marjolin ulcer: a case report and review of the literature. Wounds. 2024;36(5):166-169. doi:10.25270/wnds/23138
2. Li G, Shen Q, Zhou P, Liu H, Chen J. Acellular dermal matrix for one-stage treatment of lower extremity full-thickness skin defect: a case series. BMC Surg. 2023;23(1):17. doi:10.1186/s12893-022-01871-x
3. Baryza MJ, Baryza GA. The Vancouver Scar Scale: an administration tool and its interrater reliability. J Burn Care Rehabil. 1995;16(5):535-538. doi:10.1097/00004630-199509000-00013
4. Augustin M, Conde Montero E, Zander N, et al. Validity and feasibility of the wound-QoL questionnaire on health-related quality of life in chronic wounds. Wound Repair Regen. 2017;25(5):852-857. doi:10.1111/wrr.12583
5. Simman R, Abbas FT. Foot wounds and the reconstructive ladder. Plast Reconstr Surg Glob Open. 2021;9(12):e3989. doi:10.1097/GOX.0000000000003989
6. Crowe CS, Cho DY, Kneib CJ, Morrison SD, Friedrich JB, Keys KA. Strategies for reconstruction of the plantar surface of the foot: a systematic review of the literature. Plast Reconstr Surg. 2019;143(4):1223-1244. doi:10.1097/PRS.0000000000005448
7. Scaglioni MF, Rittirsch D, Giovanoli P. Reconstruction of the heel, middle foot sole, and plantar forefoot with the medial plantar artery perforator flap: clinical experience with 28 cases. Plast Reconstr Surg. 2018;141(1):200–208. doi:10.1097/PRS.0000000000003975
8. Pan D, Zhou ZB, Tang JY. Three-dimensional reconstruction: the waveform design of free perforator flap for the heel defect repair. Plast Reconstr Surg. 2018;142(5):809e–810e. doi:10.1097/PRS.0000000000004942
9. Santanelli F, Tenna S, Pace A, Scuderi N. Free flap reconstruction of the sole of the foot with or without sensory nerve coaptation. Plast Reconstr Surg. 2002;109(7):2314-2324. doi:10.1097/00006534-200206000-00023
10. El-Shazly M, Yassin O, Kamal A, Makboul M, Gherardini G. Soft tissue defects of the heel: a surgical reconstruction algorithm based on a retrospective cohort study. J Foot Ankle Surg. 2008;47(2):145–152. doi:10.1053/j.jfas.2007.12.010
11. Ali MA, Chowdhury P, Ali M, Zuha II, Dev J. Distally-based sural island flap for soft tissue coverage of ankle and heel defects. J Coll Physicians Surg Pak. 2010;20(7):475–477.
12. Mendieta MJ, Roblero C, Vega JC. Neurotized distally based sural flap for heel reconstruction. J Reconstr Microsurg. 2013;29(8):501–504. doi:10.1055/s-0033-1348034
13. Degeorge B, Dagneaux L, Forget D, Gaillard F, Canovas F. Delayed reconstruction by total calcaneal allograft following calcanectomy: is it an option? Case Rep Orthop. 2016;2016:4012180. doi:10.1155/2016/4012180
14. Noever G, Brüser P, Köhler L. Reconstruction of heel and sole defects by free flaps. Plast Reconstr Surg. 1986;78(3):345–352. doi:10.1097/00006534-198609000-00010
15. Torner, F, Nuñez, JH, Inarejos Clemente, EJ, et al. Total calcaneal allograft reconstruction of an Ewing's sarcoma in a child: outcome and review of the literature. Cancer Rep (Hoboken). 2022;5(9):e1626. doi:10.1002/cnr2.1626
16. Feng B, Dai GM, Wang YJ, Zhang L, Niu KC. The treatment experience of different types of flaps for repairing soft tissue defects of the heel. Int J Gen Med. 2021;14:8445-8453. doi:10.2147/IJGM.S329642
17. Krishna D, Chaturvedi G, Khan MM, Cheruvu VPR, Laitonjam M, Minz R. Reconstruction of heel soft tissue defects: an algorithm based on our experience. World J Plast Surg. 2021;10(3):63-72. doi:10.29252/wjps.10.3.63
18. Ali F, Harunarashid H, Yugasmavanan K. Delayed reverse sural flap for cover of heel defect in a patient with associated vascular injury. A case report. Indian J Surg. 2013;75(Suppl 1):148–149. doi:10.1007/s12262-012-0565-x
19. Jachna JT, Toby EB, Horton GA. Radial forearm free flap for coverage of postoperative lateral heel wounds after open reduction and internal fixation of the calcaneus. J Foot Ankle Surg. 2003;42(5):276–281. doi:10.1016/j.jfas.2003.08.001
20. Suzuki S, Matsuda K, Isshiki N, Tamada Y, Ikada Y. Experimental study of a newly developed bilayer artificial skin. Biomaterials. 1990;11(5):356–360. doi:10.1016/0142-9612(90)90114-6
21. Lembo F, Cecchino LR, Parisi D, Portincasa A. Utility of a new artificial dermis as a successful tool in face and scalp reconstruction for skin cancer: analysis of the efficacy, safety, and aesthetic outcomes. Dermatol Res Pract. 2020;2020:4874035. doi:10.1155/2020/4874035
22. Lembo F, Cecchino LR, Parisi D, Portincasa A. Role of a new acellular dermal matrix in a multistep combined treatment of dermatofibrosarcoma protuberans of the lumbar region: a case report. J Med Case Rep. 2021;15(1):180. doi:10.1186/s13256-021-02787-5
23. Lembo F, Cecchino LR, Parisi D, Portincasa A. A combined protocol for improving reconstruction of scrotal skin avulsions: the experience of the University Hospital Center of Foggia. Urologia. 2022;89(4):623-628. doi:10.1177/03915603211046164
24. Lou X, Xue H, Li G, et al. One-stage Pelnac reconstruction in full-thickness skin defects with bone or tendon exposure. Plast Reconstr Surg Glob Open. 2018;6(3):e1709. doi:10.1097/GOX.0000000000001709
25. Hao Z. Application of Pelnac® artificial dermis combined with VSD in the repair of limb wounds. J Invest Surg. 2020;33(7):642-643. doi:10.1080/08941939.2018.1551445 [Update in: Lv Z, Wang Q, Jia R, Ding W, Shen Y. Pelnac artificial dermis assisted by VSD for treatment of complex wound with bone/tendon exposed at the foot and ankle, a prospective study. J Invest Surg. 2020;33(7):636-641. doi:10.1080/08941939.2018.1536177]
26. Lv Z, Wang Q, Jia R, Ding W, Shen Y. Pelnac® artificial dermis assisted by VSD for treatment of complex wound with bone/tendon exposed at the foot and ankle, a prospective study. J Invest Surg. 2020;33(7):636-641. doi:10.1080/08941939.2018.1536177