Use of Polyhexamethylene Biguanide in the Treatment of Atopic Dermatitis With Staphylococcus Aureus Hypercolonization
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Wounds or HMP Global, their employees, and affiliates.
Abstract
Background. Atopic dermatitis (AD) is a chronic inflammatory skin disease with significant global prevalence. Clinically, AD is characterized by xerosis, intense pruritus, and recurrent eczematous lesions. The pathogenesis of AD is complex and multifactorial, involving genetic predisposition, epidermal barrier dysfunction, immune dysregulation, and dysbiosis. These factors collectively increase susceptibility to infections in patients with AD. AD lesions are frequently colonized by Staphylococcus aureus and Staphylococcus epidermidis. An important aspect of Staphylococcus spp is the propensity to form biofilms that exhibit enhanced resistance to antibiotics and host immune responses. Case Report. This report describes 2 cases of AD associated with biofilm formation that was successfully treated with polyhexamethylene biguanide (PHMB). Conclusion. This report highlights the potential of PHMB in the treatment of AD lesions and biofilm reduction.
Atopic dermatitis (AD) is a chronic inflammatory skin disease with significant global prevalence. In Brazil, the age-adjusted prevalence of reported AD ranges from 2.03% to 2.51%, with considerable variation among age groups.1 This condition typically manifests in early childhood and often leads to a substantial decline in quality of life.2 Clinically, AD is characterized by xerosis, intense pruritus, and recurrent eczematous lesions, which may present in either acute or chronic forms. The morphology of these lesions is highly heterogeneous. The classic form primarily manifests as eczema in flexural areas, whereas the nummular variant is characterized by coin-shaped lesions, predominantly located on the lower extremities and less commonly on the trunk and upper limbs.2
The pathogenesis of AD is complex and multifactorial, involving genetic predisposition, epidermal barrier dysfunction, immune dysregulation, and dysbiosis.3 These factors collectively increase susceptibility to infections in patients with AD. Infectious complications include skin and soft tissue infections, eczema herpeticum, bacteremia, osteomyelitis, septic arthritis, and endocarditis.4
AD lesions are frequently colonized by Staphylococcus aureus and Staphylococcus epidermidis. The prevalence of S aureus carriage among patients with AD is 70% for lesional skin, 39% for nonlesional skin, and 62% for the nose.5 For lesional skin, prevalence appears to be dependent on disease severity and age.5 An important feature of Staphylococcus spp is the ability to form biofilms—adhesive surface colonies that exhibit enhanced resistance to antibiotics and host immune responses.6
Polyhexamethylene biguanide (PHMB) is an antimicrobial agent commonly used in the treatment of chronic wounds due to its bactericidal and antifungal properties, with demonstrated efficacy against biofilm.7
However, no studies have reported the use of PHMB in the management of infected AD lesions complicated by biofilm formation. This report describes 2 cases of AD associated with biofilm formation that was successfully treated with PHMB.
Case Reports
Case 1
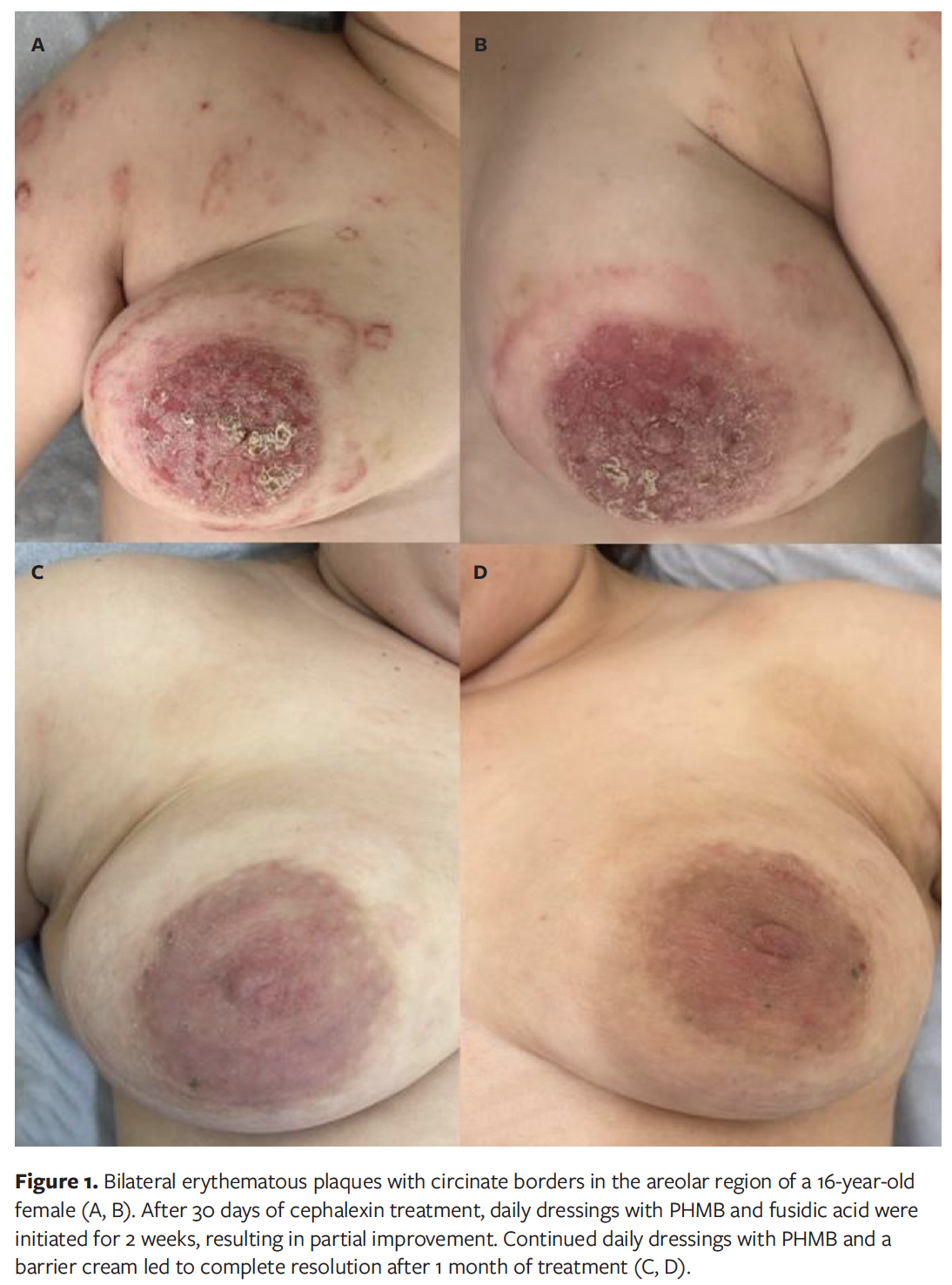
A 16-year-old female patient with a history of allergic rhinitis and generalized anxiety disorder presented with eczema localized to the areolar region, characterized by intense pruritus and sleep disturbances. Physical examination revealed erythematous, scaly plaques with exudation and well-defined, circinate borders, occurring bilaterally in the areolar region, along with some plaques on the upper extremities (Figure 1A, 1B). A diagnosis of nummular eczema with secondary bacterial infection and biofilm formation was established. The patient was initially prescribed cephalexin for 10 days, but no clinical improvement was observed. Due to the refractory nature of the condition, 30 days after completing antibiotic treatment, daily dressings with PHMB and topical fusidic acid were initiated for 14 days, in addition to decolonization measures for the patient and her family. Following treatment, the patient demonstrated partial improvement of the lesions. Continued daily dressings with PHMB combined with a barrier cream resulted in complete resolution of the lesions after 1 month of treatment (Figure 1C, 1D).
Case 2
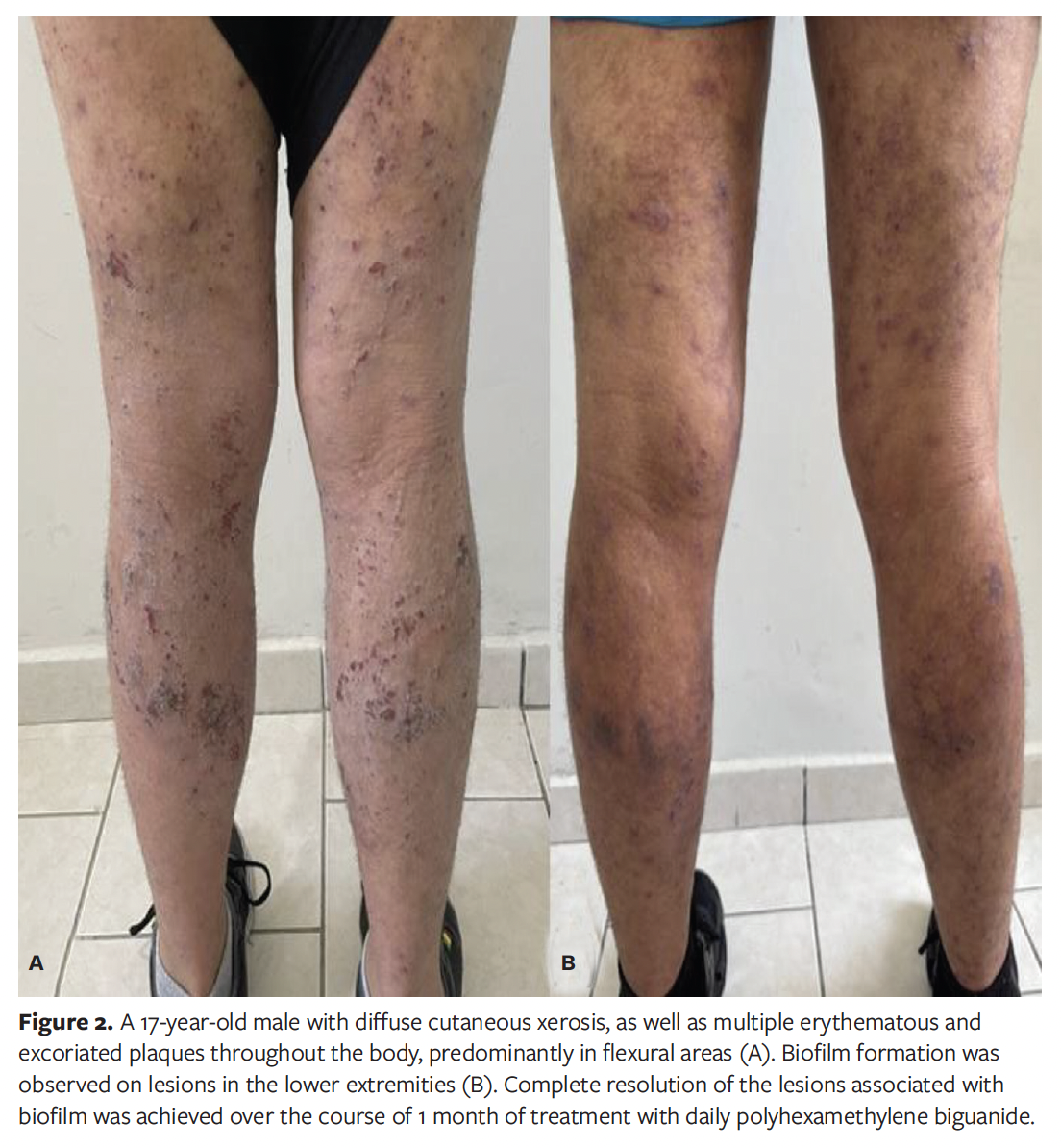
A 17-year-old male patient with a history of allergic rhinitis, asthma, anxiety disorder, and childhood-onset AD presented with diffuse cutaneous xerosis, as well as multiple erythematous and excoriated plaques throughout the body, predominantly in flexural areas. Biofilm formation was observed on lesions in the lower extremities (Figure 2A). Treatment was initiated, with daily applications of PHMB to the infected lesions. Over the course of 1 month, complete resolution of the lesions associated with biofilm was achieved (Figure 2B). Two months later, the patient presented with a positive interferon gamma release assay, was diagnosed with latent tuberculosis, and as of this writing is awaiting the initiation of systemic immunosuppressive therapy for AD, after finishing tuberculosis treatment.
Discussion
The predominance of S aureus in biofilm formation within AD lesions has been directly correlated with the severity of the disease and appears to be responsible for occlusion of sweat ducts, skin arousal, and pruritus.8 There is considerable interest in the use of antistaphylococcal agents as adjunctive therapy in AD management. Antiseptics offer an alternative adjuvant treatment for patients with infected and difficult to manage lesions. Advantages of antiseptics include their low potential to induce bacterial hyperresistance, rare occurrences of delayed-type hypersensitivity or allergic reactions, and the availability of formulations that can be tailored to individual needs.8
Antiseptics commonly used in AD include triclosan, potassium permanganate, sodium hypochlorite, and chlorhexidine gluconate.8 However, the use of PHMB in the management of AD remains limited.
PHMB is a broad-spectrum antimicrobial agent that has an effect against S aureus. First synthesized in the 1950s, PHMB is a cationic biguanide polymer that binds to negatively charged phospholipid groups of the bacterial cell wall, leading to membrane dysfunction.7 PHMB increases the distance between membrane lipid molecules and affects the proper functioning of ion pumps, various enzymes, and bacterial cell receptors.7 Furthermore, it hardens the lipid bilayer of the membrane, leading to increased permeability.7 These cumulative effects ultimately lead to rupture of the cell wall and membrane, causing the death of microorganisms exposed to the antiseptic.7 PHMB is colorless, odorless, and noncorrosive, and is soluble in water and alcohol. Its bactericidal and fungicidal action occur within 15 minutes to 30 minutes.7,9
It is widely accepted that disease outbreaks caused by secondary infection with S aureus require, and respond to, treatment with antibiotics and antiseptic agents.8 Topical antibiotics, including preparations combined with topical corticosteroids, can be used for no more than 2 weeks in patients with clinical infection in localized areas, making the use of antiseptics a complementary treatment option.8
In the present case report, it was possible to observe a significant clinical improvement in AD lesions and biofilm reduction with the use of PHMB.
Limitations
This case report has some limitations. It is a descriptive study with a small sample size, and there was previous use of antibiotics in case 1.
Conclusion
This case report highlights the potential of PHMB in the treatment of AD lesions and biofilm reduction. It is a pioneering study that provides a foundation for future research into the role of PHMB in the treatment of secondary infections in AD. Further validation through larger, randomized controlled trials is required to confirm these findings.
Author and Public Information
Authors: Gleice Freire, MD; Leticia Midori Kondo Iwamoto, MD; Tatiana de Fátima Pinto, BSN; and Caio César de Silva Castro, MD, PhD
Affiliations: Hospital de Dermatologia Sanitária do Paraná, Paraná, Brazil
Disclosures: The authors disclose no financial or other conflicts of interest.
Ethical Approval: All participants provided informed consent form for publication and associated images prior to enrollment in this study.
Correspondence: Gleice Freire, Avenue São José, 667 – Cristo Rei – Curitiba, Paraná, Brazil. 80050-350; dragleicefreire@gmail.com
Manuscript Accepted: April 21, 2025
References
1. Miot HA, Aoki V, Orfali RL, et al. The (one-year) prevalence of atopic dermatitis in Brazil: a population-based telephone survey. J Eur Acad Dermatol Venereol. Published online ahead of print March 27, 2023. doi:10.1111/jdv.19071
2. Hüppop F, Dähnhardt-Pfeiffer S, Fölster-Holst R. Characterization of classical flexural and nummular forms of atopic dermatitis in childhood with regard to anamnestic, clinical and epidermal barrier aspects. Acta Derm Venereol. 2022;102:adv00664. doi:10.2340/actadv.v101.979
3. Sroka-Tomaszewska J, Trzeciak M. Molecular mechanisms of atopic dermatitis pathogenesis. Int J Mol Sci. 2021;22(8):4130. doi:10.3390/ijms22084130
4. Wang V, Boguniewicz J, Boguniewicz M, Ong PY. The infectious complications of atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126(1):3-12. doi:10.1016/j.anai.2020.08.002
5. Totté JEE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pasmans SG. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol. 2016;175(4):687-695. doi:10.1111/bjd.14566
6. Gonzalez T, Biagini Myers JM, Herr AB, Khurana Hershey GK. Staphylococcal biofilms in atopic dermatitis. Curr Allergy Asthma Rep. 2017;17(12):81. doi:10.1007/s11882-017-0750-x
7. Babalska, ZŁ, Korbecka-Paczkowska M, Karpiński TM. Wound antiseptics and European guidelines for antiseptic application in wound treatment. Pharmaceuticals (Basel). 2021;14(12):1253. doi:10.3390/ph14121253
8. Lee M, Van Bever H. The role of antiseptic agents in atopic dermatitis. Asia Pac Allergy. 2014;4(4):230-240. doi:10.5415/apallergy.2014.4.4.230
9. Sukakul T, Dahlin J, Pontén A, et al. Contact allergy to polyhexamethylenebiguanide (polyaminopropyl biguanide). Contact Dermatitis. 2021;84(5):326-331. doi:10.1111/cod.13728
















