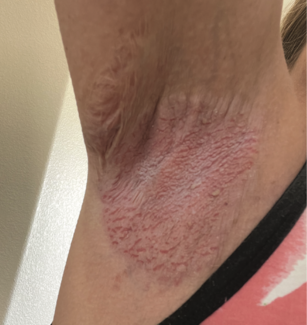
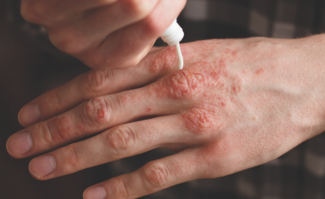
A brief review, and a look at its cost-effectiveness and insurance issues.
Herpes zoster (HZ) disease, or shingles, is a neurocutaneous syndrome involving intense pain and a unilateral, grouped vesicular eruption over one or more dermatomes.1-3 The pain may be severe, seriously impairing physical, social and emotional functioning.4 HZ results from reactivation of varicella zoster virus (VZV), which typically lies dormant in sensory ganglia after primary chickenpox infection during childhood.1, 2 The trigeminal and thoracic ganglia are most commonly involved.5 This reactivation phenomenon is associated with increasing age (> 60 years), immunosuppressive illness or treatment, and possibly family history of HZ in first-degree relatives.1,6 HZ incidence increases with age due to waning VZV-specific cell-mediated immunity.2, 7 Complications of HZ, including postherpetic neuralgia (PHN), a persistent neuropathic pain syndrome lasting for at least 30 days (90 days in some sources), also increase with age.1,3,8,9 A sizeable percentage of patients with PHN are referred to specialists for debilitating pain symptoms.10 Multiple studies have found a significantly diminished quality of life (using various scales) in those with PHN and HZ.11 As the American population ages, more cases of HZ and PHN will be seen. Currently, HZ affects between 600,000 and 1,000,000 people annually,8,12,13 more than half of whom are > 60 years of age.7,9,12 A recent study estimated incidence in those over 65 years at 19 per 1,000 population per year, with white individuals 3.5 times more likely to report HZ than Hispanic individuals.1 In a large study from Minnesota using data from 1996 to 2005, 68% of HZ cases occurred in those aged 50 years and older.14 Various studies have estimated that cumulative lifetime risk for the entire population is between 10% and 30%.5,8,9 In those over 60 years of age with HZ, PHN may occur in up to 40.8%, although the incidence is probably somewhat lower.5, 8-10 From a global perspective, the incidence and disease burden of HZ in Australia are similar to those of Europe and the United States In 1999, nearly 157,000 cases of HZ occurred in Australia, resulting in 9,161 hospital admissions.15 Data from Asia is less detailed, consisting primarily of small, hospital-based surveys. Studies from Korea and Singapore suggest that HZ epidemiology is similar to that of the United States and Europe. HZ data from South America is also poor and scant.15 Strangely enough, Brazilian studies have reported the highest incidence rates of HZ among younger adults (10 to 39 years), although Argentinean studies appear more consistent with American data.15 Nevertheless, recommendations on treatment and prevention from the United States may be relevant internationally. Herpes Zoster Vaccination in the United States A live-attenuated zoster vaccine (Zostavax, Merck & Co., Inc.) was approved by the U.S. Food and Drug Administration (FDA) on May 26, 2006.2,12 The vaccine is a higher-dose version of one currently used to prevent chickenpox in children,12 and is believed to enhance cell-mediated immunity to VZV in immunocompetent older adults.7 Results from the Shingles Prevention Study involving 38,546 subjects > 60 years of age demonstrated that vaccine reduced the burden of illness due to HZ by 61.1%, reduced the incidence of PHN by 66.5% and reduced the incidence of HZ by 51.3%. The vaccination remained effective for at least 3.12 years (the median surveillance time). Hopefully, future studies will characterize how long this boost in immunity lasts, and if higher doses of the vaccine provide greater protection.12 It is notable that vaccine-strain DNA was not detected in those who developed zoster despite vaccination.5 The vaccine is not indicated for HZ or PHN treatment (only prevention), and should not be administered to those with a history of anaphylactic or anaphylactoid reactions to gelatin or neomycin, those with a history of immunodeficiency conditions or receiving immunosuppressive therapy, those with active untreated tuberculosis or those who are pregnant.5 Side effects are primarily local and include erythema, localized pain or tenderness, swelling, pruritus, and, rarely, a varicella-like rash at the injection site (0.1%).7 The cost of vaccination is approximately $150 for a single dose, excluding the cost of administration.8,16 Merck justified this pricing, citing that shingles in the United States results in approximately $750 million in direct medical costs and $1.25 billion in indirect costs annually.12 Furthermore, in order to prevent one case of HZ or PHN, many people would need to be vaccinated.8 Thus, as for any therapeutic intervention, it is critical to examine the cost-effectiveness of zoster vaccine for society and payers. Cost-Effectiveness of VZV Vaccination in Adults As there are about 61 million Americans > 60 years of age, vaccinating all eligible Americans would cost nearly $9 billion.4 Below, we discuss studies that deal with cost-effectiveness of VZV vaccination in adults. These studies were discussed briefly in a paper by Harpaz et al, who stated, “All studies used a Markov cohort model, followed a cost-utility analytic approach that included a societal perspective, and used quality-adjusted life-year (QALY) scores to assess the incremental impact of the vaccine program on quality of life.”17 Hornberger and Robertus determined that VZV vaccination, by reducing incidence and severity of HZ, may increase quality-adjusted survival by a small amount (0.6 day) compared with no vaccination.3 Their study was conducted prior to current vaccine price establishment. They found that routine vaccination would have reasonable cost-effectiveness (vaccination costs less than $100,000 per quality-adjusted life-year gained) under the following circumstances: when (1) the unit cost of vaccination is less than $200, (2) the age at vaccination is less than 70 years (more cost-effective in “younger” older adults due to longer life expectancy and a higher level of vaccine efficacy in this group), and (3) the duration of vaccine efficacy is more than 30 years (currently unknown past 3.12 years).3 Furthermore, at a price of $50 per unit, the authors found that the vaccination would have a cost-effectiveness of < $50,000 per QALY gained. Pellissier et al presented a convincing argument for VZV vaccination in the elderly: For a representative cohort of 1,000,000 U.S. vaccine recipients 60 years of age or older, use of the vaccine could eliminate 75,000 to 89,000 HZ cases, more than 20,000 PHN cases, 10,000 HZ complications, and 40 HZ-related deaths.9 Additionally, between $82 million and $103 million in healthcare costs could be saved due to the elimination of more than 300,000 outpatient visits, 375,000 prescriptions, 9,700 ER visits, and 10,000 hospitalizations. For this cohort, the expected gain in quality-adjusted lifetime with zoster vaccine was estimated at 4,801 to 5,633 QALYs. From an economic standpoint, vaccine cost per HZ case avoided with the vaccine ranged from $1,687 to $1,985, while vaccine cost per PHN case avoided with vaccine ranged from $6,115 to $7,177. Cost-effectiveness ratios (marginal cost per QALY gained with zoster vaccine) ranged from $16,229 to $27,609 per QALY gained. Ultimately, this group judged the current vaccine price of about $150 to be cost-effective (threshold defined as $50,000 per QALY gained) for a cohort of immunocompetent U.S. vaccine recipients > 60 years old.9 They found near-equivalent cost-effectiveness and clinically preventable burden (measurement of potentially preventable loss of QALYs to the population) between zoster vaccine and cervical cancer screening (Pap smears) or cholesterol screening, based on National Commission of Prevention Practices data. Additionally, Pellissier et al found a 2.5 times higher magnitude of quality-adjusted life year gain with vaccine than did Hornberger and Robertus (0.0051-0.006 lifetime QALY gain vs. 0.0015 lifetime QALY [0.6 day] gain). They also demonstrated cost-effectiveness of zoster vaccine to be relatively insensitive to age (between 60 and 85 years).9 Reviewed by Kimberlin and Whitley, a preliminary Centers for Disease Control (CDC) and Prevention analysis found that cost per QALY ranges from $14,877 to $34,852.5. This study also found that approximately 17 people would require vaccination to prevent one case of HZ, and approximately 31 would require vaccination to prevent one case of PHN. The cost per case of HZ prevented was estimated at $3,330, while the cost per case of PHN prevented was estimated at $6,405.5 These results are consistent with those of Pellisier et al. The most recent study examining cost-effectiveness demonstrated that vaccination increased quality-adjusted life expectancy by just 0.0007 to 0.0024 QALYs per person, depending on age at vaccination and sex of patient.4 Most (50% to 65%) of the difference in quality-adjusted life expectancy was attributable to preventing PHN. Cost effectiveness, examined incrementally, ranged from $44,000 per QALY saved for a 70-year-old woman to $191,000 per QALY saved for an 80-year-old man. Generally, the cost per QALY gained ranged from $201,000 for patients aged 60 to 69 years to $75,000 for patients aged > 70 years. The model was most sensitive to vaccine cost.4 At $46 per dose, vaccination had a cost-effectiveness ratio < $50,000 per QALY saved for all adults > 60 years of age. At $150 (the real price), the vaccine did not appear to be generally cost-effective. Despite being cost-effective for 70-year-old women (given a < $50,000 per QALY threshold), it exceeded $100,000 per QALY in most other groups. Raising the threshold to $100,000 per QALY, men aged 65 to 75 years and women aged 60 to 75 years would be eligible to receive the vaccine. Rothberg et al concluded that the vaccine is more cost-effective for some recipients, particularly 70-year-old women, than for others.4 In fact, vaccinating a 70-year-old woman cost the same as vaccinating a 60-year-old man, but offered three times the health benefit. The authors call for a reduction in cost of the vaccine — at $46 per dose, it could be universally cost-effective. Insurance Coverage Unlike the tremendous success of childhood vaccinations, adult vaccinations have a far less impressive record.16 Orenstein et al discussed eight major factors for this, including insufficient financing of adult immunizations resulting in lack of incentive for healthcare providers to administer vaccinations.16 In 2005, only 4.5% of the $234,897,000 appropriated through the CDC to state and local health departments for vaccination programs (Section 317 of the Public Health Services Act) was used for adult vaccinations.16 Furthermore, the CDC estimated that close to $170 million was actually needed to purchase vaccinations for adults through the 317 program.16 In 2006, only 22 states used funds to purchase adult vaccinations, ranging from a low of $505 to a high of just $2.5 million.16 Unlike influenza vaccine, PPVS23, and hepatitis B vaccine, zoster vaccine is excluded from Medicare Part B coverage, under all circumstances.18 It is covered under most Medicare Part D drug plans, with co-payments varying depending on the plan.19 Options for physicians include: (1) writing a prescription and having the pharmacy administer the vaccine; (2) purchasing and administering the vaccine and billing the patient directly, allowing the patient to seek reimbursement from Medicare; (3) establishing relationships with pharmacists or insurance plans to reimburse them for their expenses; or (4) asking the patient to pick up the vaccine at a pharmacy and bring it to a doctor’s office — an option that is not advisable, as thermal storage requirements of the vaccine may not be followed, resulting in reduced potency).16 Not much is known about Medicaid coverage for Zostavax, although in a 2003 study of 120 Medicaid plans, the majority covered the five major vaccines for adults recommended at the time: 88% of plans covered varicella, 90% MMR, 93% influenza and PPVS23, and 95% hepatitis B.16 Private insurance plans typically cover most Advisory Committee on Immunization Practices (ACIP) vaccines recommended for adults. However, in a 2003 study, employers who self-insured were much less likely to provide immunization benefits for hepatitis B, MMR, and varicella than those who purchased commercial insurance benefits.16 In October 2006, Wellpoint, Inc., the largest health benefits company, announced it would provide complete Zostavax coverage for its members.20 Finally, the Vaccine Patient Assistance Program sponsored by Merck may provide the zoster vaccine for individuals aged 60 years and older who: (1) reside in the United States; (2) do not have health insurance; and (3) have an annual household income less than $20,800 for individuals, $28,000 for couples, or $42,400 for families of four.16, 21 However, prior to vaccine authorization from Merck, the patient’s healthcare provider must determine if the patient is an appropriate candidate, and the patient must submit an application for the vaccine.16,21 Orenstein et al eloquently outlined approaches to reduce financial barriers to adult immunizations, including increased funding for the Section 317 program and creation of a Vaccines for Uninsured Adults program. Conclusion A large study in 2005 of general internal medicine and family medicine physicians found that 35% strongly agreed that HZ and PHN caused a significant burden of disease.10 More than 80% of these physicians were somewhat or very likely to recommend the zoster vaccine to patients over 60 years of age. However, several perceived barriers were expressed, including: lack of reimbursement for vaccination, patients unwilling to pay if not covered by insurance, up-front costs to purchase the vaccine, a belief that patients will not think they need this vaccine, and insufficient information about duration of protection.10 From the time of this study, the zoster vaccine gained FDA approval and the ACIP recommendation for adults > 60 years of age (October 2006). Despite the disabling effects of HZ and PHN, it does not appear that the uptake of HZ vaccine matches the expectations set by the 2005 study. A recent CDC report found that only 2% of adults 60 years of age or older received zoster vaccination during its first year of availability.13 Under Medicare Part D, patients have to share in the cost of the HZ vaccine, unlike the widely popular influenza and PPVS23 vaccines, which are relatively inexpensive and covered by Medicare Part B.10 Schaffner suggests concomitant administration of the zoster vaccine with the influenza vaccine (as a single injection) as a means to increase use.13 Economic factors, indeed, play a large role and must be addressed to ensure widespread vaccination among our vulnerable geriatric population. Mr. Alikhan is a medical student at the University of California at Davis, School of Medicine, Sacramento, California. Dr. Maibach is Associate Professor at the University of California at San Francisco, Department of Dermatology
A brief review, and a look at its cost-effectiveness and insurance issues.
Herpes zoster (HZ) disease, or shingles, is a neurocutaneous syndrome involving intense pain and a unilateral, grouped vesicular eruption over one or more dermatomes.1-3 The pain may be severe, seriously impairing physical, social and emotional functioning.4 HZ results from reactivation of varicella zoster virus (VZV), which typically lies dormant in sensory ganglia after primary chickenpox infection during childhood.1, 2 The trigeminal and thoracic ganglia are most commonly involved.5 This reactivation phenomenon is associated with increasing age (> 60 years), immunosuppressive illness or treatment, and possibly family history of HZ in first-degree relatives.1,6 HZ incidence increases with age due to waning VZV-specific cell-mediated immunity.2, 7 Complications of HZ, including postherpetic neuralgia (PHN), a persistent neuropathic pain syndrome lasting for at least 30 days (90 days in some sources), also increase with age.1,3,8,9 A sizeable percentage of patients with PHN are referred to specialists for debilitating pain symptoms.10 Multiple studies have found a significantly diminished quality of life (using various scales) in those with PHN and HZ.11 As the American population ages, more cases of HZ and PHN will be seen. Currently, HZ affects between 600,000 and 1,000,000 people annually,8,12,13 more than half of whom are > 60 years of age.7,9,12 A recent study estimated incidence in those over 65 years at 19 per 1,000 population per year, with white individuals 3.5 times more likely to report HZ than Hispanic individuals.1 In a large study from Minnesota using data from 1996 to 2005, 68% of HZ cases occurred in those aged 50 years and older.14 Various studies have estimated that cumulative lifetime risk for the entire population is between 10% and 30%.5,8,9 In those over 60 years of age with HZ, PHN may occur in up to 40.8%, although the incidence is probably somewhat lower.5, 8-10 From a global perspective, the incidence and disease burden of HZ in Australia are similar to those of Europe and the United States In 1999, nearly 157,000 cases of HZ occurred in Australia, resulting in 9,161 hospital admissions.15 Data from Asia is less detailed, consisting primarily of small, hospital-based surveys. Studies from Korea and Singapore suggest that HZ epidemiology is similar to that of the United States and Europe. HZ data from South America is also poor and scant.15 Strangely enough, Brazilian studies have reported the highest incidence rates of HZ among younger adults (10 to 39 years), although Argentinean studies appear more consistent with American data.15 Nevertheless, recommendations on treatment and prevention from the United States may be relevant internationally. Herpes Zoster Vaccination in the United States A live-attenuated zoster vaccine (Zostavax, Merck & Co., Inc.) was approved by the U.S. Food and Drug Administration (FDA) on May 26, 2006.2,12 The vaccine is a higher-dose version of one currently used to prevent chickenpox in children,12 and is believed to enhance cell-mediated immunity to VZV in immunocompetent older adults.7 Results from the Shingles Prevention Study involving 38,546 subjects > 60 years of age demonstrated that vaccine reduced the burden of illness due to HZ by 61.1%, reduced the incidence of PHN by 66.5% and reduced the incidence of HZ by 51.3%. The vaccination remained effective for at least 3.12 years (the median surveillance time). Hopefully, future studies will characterize how long this boost in immunity lasts, and if higher doses of the vaccine provide greater protection.12 It is notable that vaccine-strain DNA was not detected in those who developed zoster despite vaccination.5 The vaccine is not indicated for HZ or PHN treatment (only prevention), and should not be administered to those with a history of anaphylactic or anaphylactoid reactions to gelatin or neomycin, those with a history of immunodeficiency conditions or receiving immunosuppressive therapy, those with active untreated tuberculosis or those who are pregnant.5 Side effects are primarily local and include erythema, localized pain or tenderness, swelling, pruritus, and, rarely, a varicella-like rash at the injection site (0.1%).7 The cost of vaccination is approximately $150 for a single dose, excluding the cost of administration.8,16 Merck justified this pricing, citing that shingles in the United States results in approximately $750 million in direct medical costs and $1.25 billion in indirect costs annually.12 Furthermore, in order to prevent one case of HZ or PHN, many people would need to be vaccinated.8 Thus, as for any therapeutic intervention, it is critical to examine the cost-effectiveness of zoster vaccine for society and payers. Cost-Effectiveness of VZV Vaccination in Adults As there are about 61 million Americans > 60 years of age, vaccinating all eligible Americans would cost nearly $9 billion.4 Below, we discuss studies that deal with cost-effectiveness of VZV vaccination in adults. These studies were discussed briefly in a paper by Harpaz et al, who stated, “All studies used a Markov cohort model, followed a cost-utility analytic approach that included a societal perspective, and used quality-adjusted life-year (QALY) scores to assess the incremental impact of the vaccine program on quality of life.”17 Hornberger and Robertus determined that VZV vaccination, by reducing incidence and severity of HZ, may increase quality-adjusted survival by a small amount (0.6 day) compared with no vaccination.3 Their study was conducted prior to current vaccine price establishment. They found that routine vaccination would have reasonable cost-effectiveness (vaccination costs less than $100,000 per quality-adjusted life-year gained) under the following circumstances: when (1) the unit cost of vaccination is less than $200, (2) the age at vaccination is less than 70 years (more cost-effective in “younger” older adults due to longer life expectancy and a higher level of vaccine efficacy in this group), and (3) the duration of vaccine efficacy is more than 30 years (currently unknown past 3.12 years).3 Furthermore, at a price of $50 per unit, the authors found that the vaccination would have a cost-effectiveness of < $50,000 per QALY gained. Pellissier et al presented a convincing argument for VZV vaccination in the elderly: For a representative cohort of 1,000,000 U.S. vaccine recipients 60 years of age or older, use of the vaccine could eliminate 75,000 to 89,000 HZ cases, more than 20,000 PHN cases, 10,000 HZ complications, and 40 HZ-related deaths.9 Additionally, between $82 million and $103 million in healthcare costs could be saved due to the elimination of more than 300,000 outpatient visits, 375,000 prescriptions, 9,700 ER visits, and 10,000 hospitalizations. For this cohort, the expected gain in quality-adjusted lifetime with zoster vaccine was estimated at 4,801 to 5,633 QALYs. From an economic standpoint, vaccine cost per HZ case avoided with the vaccine ranged from $1,687 to $1,985, while vaccine cost per PHN case avoided with vaccine ranged from $6,115 to $7,177. Cost-effectiveness ratios (marginal cost per QALY gained with zoster vaccine) ranged from $16,229 to $27,609 per QALY gained. Ultimately, this group judged the current vaccine price of about $150 to be cost-effective (threshold defined as $50,000 per QALY gained) for a cohort of immunocompetent U.S. vaccine recipients > 60 years old.9 They found near-equivalent cost-effectiveness and clinically preventable burden (measurement of potentially preventable loss of QALYs to the population) between zoster vaccine and cervical cancer screening (Pap smears) or cholesterol screening, based on National Commission of Prevention Practices data. Additionally, Pellissier et al found a 2.5 times higher magnitude of quality-adjusted life year gain with vaccine than did Hornberger and Robertus (0.0051-0.006 lifetime QALY gain vs. 0.0015 lifetime QALY [0.6 day] gain). They also demonstrated cost-effectiveness of zoster vaccine to be relatively insensitive to age (between 60 and 85 years).9 Reviewed by Kimberlin and Whitley, a preliminary Centers for Disease Control (CDC) and Prevention analysis found that cost per QALY ranges from $14,877 to $34,852.5. This study also found that approximately 17 people would require vaccination to prevent one case of HZ, and approximately 31 would require vaccination to prevent one case of PHN. The cost per case of HZ prevented was estimated at $3,330, while the cost per case of PHN prevented was estimated at $6,405.5 These results are consistent with those of Pellisier et al. The most recent study examining cost-effectiveness demonstrated that vaccination increased quality-adjusted life expectancy by just 0.0007 to 0.0024 QALYs per person, depending on age at vaccination and sex of patient.4 Most (50% to 65%) of the difference in quality-adjusted life expectancy was attributable to preventing PHN. Cost effectiveness, examined incrementally, ranged from $44,000 per QALY saved for a 70-year-old woman to $191,000 per QALY saved for an 80-year-old man. Generally, the cost per QALY gained ranged from $201,000 for patients aged 60 to 69 years to $75,000 for patients aged > 70 years. The model was most sensitive to vaccine cost.4 At $46 per dose, vaccination had a cost-effectiveness ratio < $50,000 per QALY saved for all adults > 60 years of age. At $150 (the real price), the vaccine did not appear to be generally cost-effective. Despite being cost-effective for 70-year-old women (given a < $50,000 per QALY threshold), it exceeded $100,000 per QALY in most other groups. Raising the threshold to $100,000 per QALY, men aged 65 to 75 years and women aged 60 to 75 years would be eligible to receive the vaccine. Rothberg et al concluded that the vaccine is more cost-effective for some recipients, particularly 70-year-old women, than for others.4 In fact, vaccinating a 70-year-old woman cost the same as vaccinating a 60-year-old man, but offered three times the health benefit. The authors call for a reduction in cost of the vaccine — at $46 per dose, it could be universally cost-effective. Insurance Coverage Unlike the tremendous success of childhood vaccinations, adult vaccinations have a far less impressive record.16 Orenstein et al discussed eight major factors for this, including insufficient financing of adult immunizations resulting in lack of incentive for healthcare providers to administer vaccinations.16 In 2005, only 4.5% of the $234,897,000 appropriated through the CDC to state and local health departments for vaccination programs (Section 317 of the Public Health Services Act) was used for adult vaccinations.16 Furthermore, the CDC estimated that close to $170 million was actually needed to purchase vaccinations for adults through the 317 program.16 In 2006, only 22 states used funds to purchase adult vaccinations, ranging from a low of $505 to a high of just $2.5 million.16 Unlike influenza vaccine, PPVS23, and hepatitis B vaccine, zoster vaccine is excluded from Medicare Part B coverage, under all circumstances.18 It is covered under most Medicare Part D drug plans, with co-payments varying depending on the plan.19 Options for physicians include: (1) writing a prescription and having the pharmacy administer the vaccine; (2) purchasing and administering the vaccine and billing the patient directly, allowing the patient to seek reimbursement from Medicare; (3) establishing relationships with pharmacists or insurance plans to reimburse them for their expenses; or (4) asking the patient to pick up the vaccine at a pharmacy and bring it to a doctor’s office — an option that is not advisable, as thermal storage requirements of the vaccine may not be followed, resulting in reduced potency).16 Not much is known about Medicaid coverage for Zostavax, although in a 2003 study of 120 Medicaid plans, the majority covered the five major vaccines for adults recommended at the time: 88% of plans covered varicella, 90% MMR, 93% influenza and PPVS23, and 95% hepatitis B.16 Private insurance plans typically cover most Advisory Committee on Immunization Practices (ACIP) vaccines recommended for adults. However, in a 2003 study, employers who self-insured were much less likely to provide immunization benefits for hepatitis B, MMR, and varicella than those who purchased commercial insurance benefits.16 In October 2006, Wellpoint, Inc., the largest health benefits company, announced it would provide complete Zostavax coverage for its members.20 Finally, the Vaccine Patient Assistance Program sponsored by Merck may provide the zoster vaccine for individuals aged 60 years and older who: (1) reside in the United States; (2) do not have health insurance; and (3) have an annual household income less than $20,800 for individuals, $28,000 for couples, or $42,400 for families of four.16, 21 However, prior to vaccine authorization from Merck, the patient’s healthcare provider must determine if the patient is an appropriate candidate, and the patient must submit an application for the vaccine.16,21 Orenstein et al eloquently outlined approaches to reduce financial barriers to adult immunizations, including increased funding for the Section 317 program and creation of a Vaccines for Uninsured Adults program. Conclusion A large study in 2005 of general internal medicine and family medicine physicians found that 35% strongly agreed that HZ and PHN caused a significant burden of disease.10 More than 80% of these physicians were somewhat or very likely to recommend the zoster vaccine to patients over 60 years of age. However, several perceived barriers were expressed, including: lack of reimbursement for vaccination, patients unwilling to pay if not covered by insurance, up-front costs to purchase the vaccine, a belief that patients will not think they need this vaccine, and insufficient information about duration of protection.10 From the time of this study, the zoster vaccine gained FDA approval and the ACIP recommendation for adults > 60 years of age (October 2006). Despite the disabling effects of HZ and PHN, it does not appear that the uptake of HZ vaccine matches the expectations set by the 2005 study. A recent CDC report found that only 2% of adults 60 years of age or older received zoster vaccination during its first year of availability.13 Under Medicare Part D, patients have to share in the cost of the HZ vaccine, unlike the widely popular influenza and PPVS23 vaccines, which are relatively inexpensive and covered by Medicare Part B.10 Schaffner suggests concomitant administration of the zoster vaccine with the influenza vaccine (as a single injection) as a means to increase use.13 Economic factors, indeed, play a large role and must be addressed to ensure widespread vaccination among our vulnerable geriatric population. Mr. Alikhan is a medical student at the University of California at Davis, School of Medicine, Sacramento, California. Dr. Maibach is Associate Professor at the University of California at San Francisco, Department of Dermatology
A brief review, and a look at its cost-effectiveness and insurance issues.
Herpes zoster (HZ) disease, or shingles, is a neurocutaneous syndrome involving intense pain and a unilateral, grouped vesicular eruption over one or more dermatomes.1-3 The pain may be severe, seriously impairing physical, social and emotional functioning.4 HZ results from reactivation of varicella zoster virus (VZV), which typically lies dormant in sensory ganglia after primary chickenpox infection during childhood.1, 2 The trigeminal and thoracic ganglia are most commonly involved.5 This reactivation phenomenon is associated with increasing age (> 60 years), immunosuppressive illness or treatment, and possibly family history of HZ in first-degree relatives.1,6 HZ incidence increases with age due to waning VZV-specific cell-mediated immunity.2, 7 Complications of HZ, including postherpetic neuralgia (PHN), a persistent neuropathic pain syndrome lasting for at least 30 days (90 days in some sources), also increase with age.1,3,8,9 A sizeable percentage of patients with PHN are referred to specialists for debilitating pain symptoms.10 Multiple studies have found a significantly diminished quality of life (using various scales) in those with PHN and HZ.11 As the American population ages, more cases of HZ and PHN will be seen. Currently, HZ affects between 600,000 and 1,000,000 people annually,8,12,13 more than half of whom are > 60 years of age.7,9,12 A recent study estimated incidence in those over 65 years at 19 per 1,000 population per year, with white individuals 3.5 times more likely to report HZ than Hispanic individuals.1 In a large study from Minnesota using data from 1996 to 2005, 68% of HZ cases occurred in those aged 50 years and older.14 Various studies have estimated that cumulative lifetime risk for the entire population is between 10% and 30%.5,8,9 In those over 60 years of age with HZ, PHN may occur in up to 40.8%, although the incidence is probably somewhat lower.5, 8-10 From a global perspective, the incidence and disease burden of HZ in Australia are similar to those of Europe and the United States In 1999, nearly 157,000 cases of HZ occurred in Australia, resulting in 9,161 hospital admissions.15 Data from Asia is less detailed, consisting primarily of small, hospital-based surveys. Studies from Korea and Singapore suggest that HZ epidemiology is similar to that of the United States and Europe. HZ data from South America is also poor and scant.15 Strangely enough, Brazilian studies have reported the highest incidence rates of HZ among younger adults (10 to 39 years), although Argentinean studies appear more consistent with American data.15 Nevertheless, recommendations on treatment and prevention from the United States may be relevant internationally. Herpes Zoster Vaccination in the United States A live-attenuated zoster vaccine (Zostavax, Merck & Co., Inc.) was approved by the U.S. Food and Drug Administration (FDA) on May 26, 2006.2,12 The vaccine is a higher-dose version of one currently used to prevent chickenpox in children,12 and is believed to enhance cell-mediated immunity to VZV in immunocompetent older adults.7 Results from the Shingles Prevention Study involving 38,546 subjects > 60 years of age demonstrated that vaccine reduced the burden of illness due to HZ by 61.1%, reduced the incidence of PHN by 66.5% and reduced the incidence of HZ by 51.3%. The vaccination remained effective for at least 3.12 years (the median surveillance time). Hopefully, future studies will characterize how long this boost in immunity lasts, and if higher doses of the vaccine provide greater protection.12 It is notable that vaccine-strain DNA was not detected in those who developed zoster despite vaccination.5 The vaccine is not indicated for HZ or PHN treatment (only prevention), and should not be administered to those with a history of anaphylactic or anaphylactoid reactions to gelatin or neomycin, those with a history of immunodeficiency conditions or receiving immunosuppressive therapy, those with active untreated tuberculosis or those who are pregnant.5 Side effects are primarily local and include erythema, localized pain or tenderness, swelling, pruritus, and, rarely, a varicella-like rash at the injection site (0.1%).7 The cost of vaccination is approximately $150 for a single dose, excluding the cost of administration.8,16 Merck justified this pricing, citing that shingles in the United States results in approximately $750 million in direct medical costs and $1.25 billion in indirect costs annually.12 Furthermore, in order to prevent one case of HZ or PHN, many people would need to be vaccinated.8 Thus, as for any therapeutic intervention, it is critical to examine the cost-effectiveness of zoster vaccine for society and payers. Cost-Effectiveness of VZV Vaccination in Adults As there are about 61 million Americans > 60 years of age, vaccinating all eligible Americans would cost nearly $9 billion.4 Below, we discuss studies that deal with cost-effectiveness of VZV vaccination in adults. These studies were discussed briefly in a paper by Harpaz et al, who stated, “All studies used a Markov cohort model, followed a cost-utility analytic approach that included a societal perspective, and used quality-adjusted life-year (QALY) scores to assess the incremental impact of the vaccine program on quality of life.”17 Hornberger and Robertus determined that VZV vaccination, by reducing incidence and severity of HZ, may increase quality-adjusted survival by a small amount (0.6 day) compared with no vaccination.3 Their study was conducted prior to current vaccine price establishment. They found that routine vaccination would have reasonable cost-effectiveness (vaccination costs less than $100,000 per quality-adjusted life-year gained) under the following circumstances: when (1) the unit cost of vaccination is less than $200, (2) the age at vaccination is less than 70 years (more cost-effective in “younger” older adults due to longer life expectancy and a higher level of vaccine efficacy in this group), and (3) the duration of vaccine efficacy is more than 30 years (currently unknown past 3.12 years).3 Furthermore, at a price of $50 per unit, the authors found that the vaccination would have a cost-effectiveness of < $50,000 per QALY gained. Pellissier et al presented a convincing argument for VZV vaccination in the elderly: For a representative cohort of 1,000,000 U.S. vaccine recipients 60 years of age or older, use of the vaccine could eliminate 75,000 to 89,000 HZ cases, more than 20,000 PHN cases, 10,000 HZ complications, and 40 HZ-related deaths.9 Additionally, between $82 million and $103 million in healthcare costs could be saved due to the elimination of more than 300,000 outpatient visits, 375,000 prescriptions, 9,700 ER visits, and 10,000 hospitalizations. For this cohort, the expected gain in quality-adjusted lifetime with zoster vaccine was estimated at 4,801 to 5,633 QALYs. From an economic standpoint, vaccine cost per HZ case avoided with the vaccine ranged from $1,687 to $1,985, while vaccine cost per PHN case avoided with vaccine ranged from $6,115 to $7,177. Cost-effectiveness ratios (marginal cost per QALY gained with zoster vaccine) ranged from $16,229 to $27,609 per QALY gained. Ultimately, this group judged the current vaccine price of about $150 to be cost-effective (threshold defined as $50,000 per QALY gained) for a cohort of immunocompetent U.S. vaccine recipients > 60 years old.9 They found near-equivalent cost-effectiveness and clinically preventable burden (measurement of potentially preventable loss of QALYs to the population) between zoster vaccine and cervical cancer screening (Pap smears) or cholesterol screening, based on National Commission of Prevention Practices data. Additionally, Pellissier et al found a 2.5 times higher magnitude of quality-adjusted life year gain with vaccine than did Hornberger and Robertus (0.0051-0.006 lifetime QALY gain vs. 0.0015 lifetime QALY [0.6 day] gain). They also demonstrated cost-effectiveness of zoster vaccine to be relatively insensitive to age (between 60 and 85 years).9 Reviewed by Kimberlin and Whitley, a preliminary Centers for Disease Control (CDC) and Prevention analysis found that cost per QALY ranges from $14,877 to $34,852.5. This study also found that approximately 17 people would require vaccination to prevent one case of HZ, and approximately 31 would require vaccination to prevent one case of PHN. The cost per case of HZ prevented was estimated at $3,330, while the cost per case of PHN prevented was estimated at $6,405.5 These results are consistent with those of Pellisier et al. The most recent study examining cost-effectiveness demonstrated that vaccination increased quality-adjusted life expectancy by just 0.0007 to 0.0024 QALYs per person, depending on age at vaccination and sex of patient.4 Most (50% to 65%) of the difference in quality-adjusted life expectancy was attributable to preventing PHN. Cost effectiveness, examined incrementally, ranged from $44,000 per QALY saved for a 70-year-old woman to $191,000 per QALY saved for an 80-year-old man. Generally, the cost per QALY gained ranged from $201,000 for patients aged 60 to 69 years to $75,000 for patients aged > 70 years. The model was most sensitive to vaccine cost.4 At $46 per dose, vaccination had a cost-effectiveness ratio < $50,000 per QALY saved for all adults > 60 years of age. At $150 (the real price), the vaccine did not appear to be generally cost-effective. Despite being cost-effective for 70-year-old women (given a < $50,000 per QALY threshold), it exceeded $100,000 per QALY in most other groups. Raising the threshold to $100,000 per QALY, men aged 65 to 75 years and women aged 60 to 75 years would be eligible to receive the vaccine. Rothberg et al concluded that the vaccine is more cost-effective for some recipients, particularly 70-year-old women, than for others.4 In fact, vaccinating a 70-year-old woman cost the same as vaccinating a 60-year-old man, but offered three times the health benefit. The authors call for a reduction in cost of the vaccine — at $46 per dose, it could be universally cost-effective. Insurance Coverage Unlike the tremendous success of childhood vaccinations, adult vaccinations have a far less impressive record.16 Orenstein et al discussed eight major factors for this, including insufficient financing of adult immunizations resulting in lack of incentive for healthcare providers to administer vaccinations.16 In 2005, only 4.5% of the $234,897,000 appropriated through the CDC to state and local health departments for vaccination programs (Section 317 of the Public Health Services Act) was used for adult vaccinations.16 Furthermore, the CDC estimated that close to $170 million was actually needed to purchase vaccinations for adults through the 317 program.16 In 2006, only 22 states used funds to purchase adult vaccinations, ranging from a low of $505 to a high of just $2.5 million.16 Unlike influenza vaccine, PPVS23, and hepatitis B vaccine, zoster vaccine is excluded from Medicare Part B coverage, under all circumstances.18 It is covered under most Medicare Part D drug plans, with co-payments varying depending on the plan.19 Options for physicians include: (1) writing a prescription and having the pharmacy administer the vaccine; (2) purchasing and administering the vaccine and billing the patient directly, allowing the patient to seek reimbursement from Medicare; (3) establishing relationships with pharmacists or insurance plans to reimburse them for their expenses; or (4) asking the patient to pick up the vaccine at a pharmacy and bring it to a doctor’s office — an option that is not advisable, as thermal storage requirements of the vaccine may not be followed, resulting in reduced potency).16 Not much is known about Medicaid coverage for Zostavax, although in a 2003 study of 120 Medicaid plans, the majority covered the five major vaccines for adults recommended at the time: 88% of plans covered varicella, 90% MMR, 93% influenza and PPVS23, and 95% hepatitis B.16 Private insurance plans typically cover most Advisory Committee on Immunization Practices (ACIP) vaccines recommended for adults. However, in a 2003 study, employers who self-insured were much less likely to provide immunization benefits for hepatitis B, MMR, and varicella than those who purchased commercial insurance benefits.16 In October 2006, Wellpoint, Inc., the largest health benefits company, announced it would provide complete Zostavax coverage for its members.20 Finally, the Vaccine Patient Assistance Program sponsored by Merck may provide the zoster vaccine for individuals aged 60 years and older who: (1) reside in the United States; (2) do not have health insurance; and (3) have an annual household income less than $20,800 for individuals, $28,000 for couples, or $42,400 for families of four.16, 21 However, prior to vaccine authorization from Merck, the patient’s healthcare provider must determine if the patient is an appropriate candidate, and the patient must submit an application for the vaccine.16,21 Orenstein et al eloquently outlined approaches to reduce financial barriers to adult immunizations, including increased funding for the Section 317 program and creation of a Vaccines for Uninsured Adults program. Conclusion A large study in 2005 of general internal medicine and family medicine physicians found that 35% strongly agreed that HZ and PHN caused a significant burden of disease.10 More than 80% of these physicians were somewhat or very likely to recommend the zoster vaccine to patients over 60 years of age. However, several perceived barriers were expressed, including: lack of reimbursement for vaccination, patients unwilling to pay if not covered by insurance, up-front costs to purchase the vaccine, a belief that patients will not think they need this vaccine, and insufficient information about duration of protection.10 From the time of this study, the zoster vaccine gained FDA approval and the ACIP recommendation for adults > 60 years of age (October 2006). Despite the disabling effects of HZ and PHN, it does not appear that the uptake of HZ vaccine matches the expectations set by the 2005 study. A recent CDC report found that only 2% of adults 60 years of age or older received zoster vaccination during its first year of availability.13 Under Medicare Part D, patients have to share in the cost of the HZ vaccine, unlike the widely popular influenza and PPVS23 vaccines, which are relatively inexpensive and covered by Medicare Part B.10 Schaffner suggests concomitant administration of the zoster vaccine with the influenza vaccine (as a single injection) as a means to increase use.13 Economic factors, indeed, play a large role and must be addressed to ensure widespread vaccination among our vulnerable geriatric population. Mr. Alikhan is a medical student at the University of California at Davis, School of Medicine, Sacramento, California. Dr. Maibach is Associate Professor at the University of California at San Francisco, Department of Dermatology