FDA Approves Roflumilast Cream 0.05% for Treatment of Atopic Dermatitis in Children Aged 2 to 5
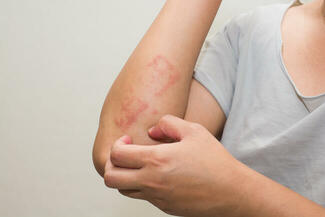
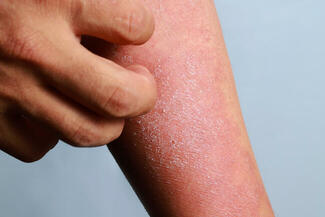
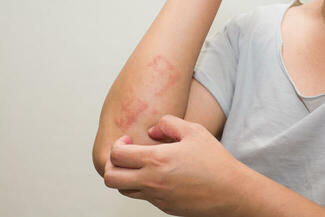
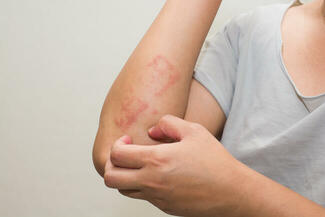
On October 6, 2025, the US Food and Drug Administration (FDA) approved ZORYVE (roflumilast) cream 0.05% for the topical treatment of mild-to-moderate atopic dermatitis (AD) in pediatric patients aged 2 to 5 years. This once-daily, steroid-free cream offers rapid, effective, and well-tolerated relief and can be used anywhere on the body for any duration.
“In clinical trials, roflumilast cream 0.05% rapidly improved the severity and extent of eczema (atopic dermatitis) signs and symptoms, with approximately 40% of children achieving a 75% improvement from baseline as measured by EASI, and more than one-third (35%) achieving a clinically meaningful improvement in itch intensity, both in only 4 weeks,” said Lawrence F. Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego and INTEGUMENT study investigator.
The FDA approval is based on data from the phase 3 INTEGUMENT-PED trial, supported by the long-term INTEGUMENT-OLE extension study and a phase 1 pharmacokinetic study. The INTEGUMENT-PED trial enrolled 652 children aged 2 to 5 years with mild-to-moderate AD, randomized to receive either roflumilast cream 0.05% or vehicle once daily for 4 weeks.
At week 4, the study demonstrated:
- 25.4% of children using roflumilast achieved validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) success vs 10.7% with vehicle (P<0.0001)
- 39.4% of roflumilast-treated patients achieved Eczema Area and Severity Index (EASI)-75 vs 20.0% with vehicle
- Over one-third achieved a 4-point reduction in Worst Itch Numeric Rating Scale vs 18% with vehicle (nominal P=0.0002)
- Disease clearance improvements were observed as early as week 1
vIGA-AD success was defined as a score of clear or almost clear with a ≥2-grade improvement from baseline. In exploratory endpoints, caregivers reported itch relief in some children within 24 hours of the first application.
The most common adverse events with roflumilast cream 0.05% were upper respiratory tract infection, diarrhea, vomiting, rhinitis, conjunctivitis, and headache. No new safety concerns emerged during the trial. The safety profile remained consistent throughout the 56-week INTEGUMENT-OLE study, where 71.9% of patients achieved EASI-75 by the end of long-term treatment.
“Young children are particularly vulnerable to the bothersome symptoms of atopic dermatitis, because their immune system and skin barrier are less developed than those of older children and adults,” said Korey Capozza, MPH, founder and executive director at Global Parents for Eczema Research. “This condition doesn’t just affect the child’s skin — it can affect the whole family by causing sleep disruption, emotional distress, and social isolation.”
With this latest indication, ZORYVE now holds 6 FDA approvals in just over 3 years, including AD in patients aged 2 and older, and plaque psoriasis and seborrheic dermatitis in older children and adults. ZORYVE is an advanced phosphodiesterase 4 inhibitor designed to target underlying inflammation without compromising the skin barrier. Roflumilast cream 0.05% is expected to be commercially available by the end of October 2025.
Reference
FDA approves Arcutis’ ZORYVE (roflumilast) cream 0.05% for the treatment of atopic dermatitis in children ages 2 to 5. News release. October 6, 2025. Accessed October 6, 2025. https://investors.arcutis.com/news-releases/news-release-details/fda-approves-arcutis-zoryver-roflumilast-cream-005-treatment