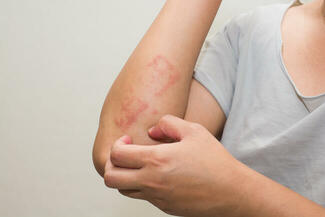
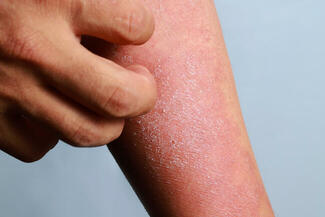
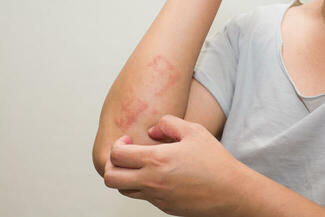
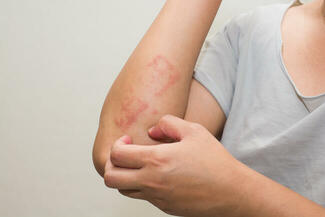
FDA Approves Opzelura for Atopic Dermatitis in Children Aged 2–11
On September 18, 2025, the US Food and Drug Administration (FDA) approved Opzelura (ruxolitinib) cream 1.5% for the short-term and noncontinuous chronic treatment of mild-to-moderate atopic dermatitis (AD) in nonimmunocompromised children aged 2 years and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
“With this approval, we are now able to offer younger children with atopic dermatitis and their families a much-needed, steroid-free topical treatment option with the potential to significantly improve the burdensome symptoms they experience every day,” said Incyte CEO Bill Meury.
The FDA approval is based on data from the pivotal phase 3 TRuE-AD3 trial, which evaluated Opzelura in over 300 pediatric patients aged 2 to <12 years with mild-to-moderate AD. Patients were randomized to receive ruxolitinib cream 0.75%, ruxolitinib cream 1.5%, or a vehicle (nonmedicated cream), applied twice daily for 8 weeks.
At week 8, the study demonstrated:
- Significantly more patients treated with Opzelura achieved Investigator’s Global Assessment–Treatment Success (IGA-TS) compared to the vehicle
- A higher proportion of patients achieved ≥75% improvement in Eczema Area and Severity Index (EASI75) scores
- Improvements in itch severity were also observed in children aged 6 to <12 years
IGA-TS was defined as a score of 0 (clear) or 1 (almost clear) with at least a 2-point improvement from baseline. Patients who completed the 8-week treatment phase were offered enrollment in a 44-week long-term extension.
The most common adverse reaction was upper respiratory tract infection. No serious infections, malignancies, major adverse cardiovascular events, or thromboses were reported during the vehicle-controlled period. Overall, the safety profile of Opzelura in children was consistent with previous studies in older populations.
“Navigating a complex condition like atopic dermatitis can be very challenging for children, who currently have limited treatment options to meet their specific needs,” said Dr Peter Lio, clinical assistant professor of dermatology and pediatrics at Northwestern University Feinberg School of Medicine. “With this approval, we now have a new, nonsteroidal topical option that expands how we care for kids with this chronic disease. This is a meaningful step forward and marks a significant advancement in our ability to better support our pediatric patients.”
With this latest indication, Opzelura is now approved in the United States for the treatment of mild-to-moderate AD in patients aged 2 years and older and the treatment of nonsegmental vitiligo in patients aged 12 years and older.
Opzelura is administered as a topical cream applied twice daily and may be used in a clinical setting or at home under the supervision of a health care provider. It is not recommended for use in combination with therapeutic biologics, other Janus kinase inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine.
Reference
Incyte announces additional FDA approval of Opzelura (ruxolitinib) cream in children ages 2–11 with atopic dermatitis. Press release. Incyte. September 18, 2025. Accessed September 18, 2025. https://investor.incyte.com/news-releases/news-release-details/incyte-announces-new-data-ruxolitinib-cream-opzelurar-children