What Caused This Alopecic Lesion on the Scalp?
Case Report
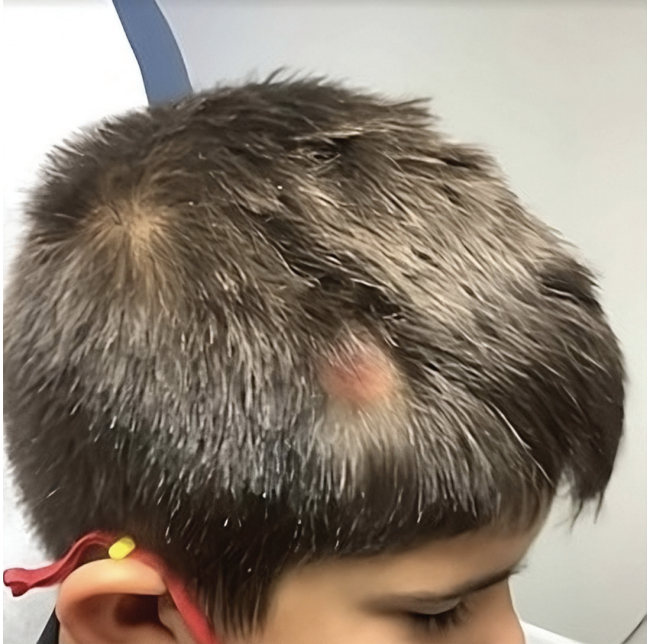
An 8-year-old boy presented with a slightly pruritic nodule with an overlying alopecic patch on his right frontal scalp (Figure). The lesion appeared a few months prior and was evaluated by his primary care physician who diagnosed the patch as a kerion of the scalp and prescribed an oral antibiotic and topical ketoconazole with minimal improvement. The patient’s parents reported the lesion started as a small alopecic patch that slowly evolved into a pinkish-colored, raised lesion underneath over time. There was no reported history of trauma or arthropod bites. Physical examination revealed an alopecic area overlying a poorly circumscribed, non-encapsulated nodule measuring 2.1 cm x 1.1cm on the right frontal scalp with pink background without ulceration and scaling. An excisional biopsy was recommended for both diagnostic and therapeutic purposes.
What is your diagnosis?
Check your answer below
Diagnosis:
Pseudolymphoma of the Scalp
Histologic examination of the lesion showed deep dermal and subcutaneous involvement of the skin with dense nodular and diffuse infiltrates of variably sized lymphoid cells, admixed with histiocytes, scattered eosinophils, and plasma cells. Well-formed secondary lymphoid follicles with germinal centers were noted throughout the infiltrates without atypical cells. No exogenous material was identified in the lesions, such as hypostome of the tick or tattoo pigment, and the overlying epidermis was unremarkable. Immunohistochemical (IHC) studies showed aggregates of CD20-positive B cells, mainly in follicular structures, admixed with numerous T cells that were CD3, CD5, and CD43 positive. CD23 and CD21 stains highlighted many of the B cells in the nodular areas where germinal centers were seen. Polymerase chain reaction (PCR) clonality studies were negative. Overall, IHC and PCR studies confirmed the diagnosis of B-cell pseudolymphoma. Pseudolymphoma (also known as cutaneous pseudolymphoma, cutaneous lymphoid hyperplasia, lymphocytoma cutis, lymphadenosis benigna cutis, and Spiegler-Fendt sarcoid) is a benign lymphoid reaction that results from various antigenic stimuli, which may have the potential for progression to overt lymphoma.1 It was first described by Kaposi under the term of sarcomatosis cutis. Pseudolymphoma (PSL) was first used in 1963 in an article referring to pulmonary pseudolymphoma.2
Clinical Presentation
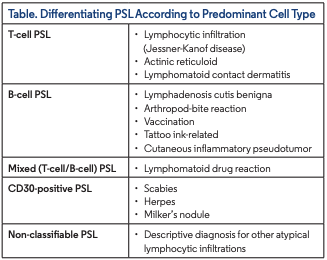
PSL presents with heterogenous clinical findings and is categorized in 4 main groups: nodular PSL, pseudo-mycosis fungoides, other PSLs (representing distinct clinical entities reported as PSL), and intravascular PSL. Clinical presentation ranges from a solitary nodule to clustered or disseminated papules, and rarely erythroderma. The most common presentation is solitary nodular lesions simulating cutaneous B-cell and T-cell lymphomas both histologically and clinically. Histologically, nodular PSL can be classified into B-cell, T-cell and mixed (T-cell/B-cell) PSL, although this classification is controversial because T-cell PSL always contains B cells and vice versa. The histologic analysis plays a crucial role in diagnosing PSL and distinguishing this entity from malignant counterparts3 (Table).
Nodular PSL presents with solitary nodules up to 4 cm in diameter in most cases, one-third being generalized, either aggregated or clustered as disseminated papules. The face, nose, cheeks, upper trunk, and arms are the most commonly involved sites. The majority of cases are in patients younger than age 40 years, less than 10% being children and adolescents, with a 3:1 male-to-female ratio.4,5 Although a wide range of causative agents, including trauma, insect bite, spirochetal and viral infections, tattooing, vaccination, piercing, drugs, and photosensitivity have been reported, the trigger is not identified in the most cases.6
Alopecia can be seen in the setting of autoimmune, infectious, neoplastic, and inflammatory disorders. Alopecia secondary to PSL has been reported in a few cases in the literature. Castelli et al. reported 4 cases of PSL located on the scalp in children between the ages of 6 and 12 years, with 2 of the cases associated with a tick bite.7 Taguchi et al. reported a case of a 58-year-old woman with erythematous plaques that caused alopecia and biopsy was consistent with PSL; however, this patient did not have a history of tick bite to the area.8 The exact mechanism of PSL-induced alopecia is unknown. Possible mechanisms of alopecia secondary to neoplasms have been attributed to fibrosis of the tissue,9 mechanical pressure by neoplastic cells to the follicles,10 and hair cycle arrest secondary to unknown mechanisms.11,12 Further investigations are needed to elucidate such relationships.
Histopathology
Histologically, nodular PSL is characterized by dense nodular infiltrate located in the reticular dermis extending to superficial parts of the subcutis. The infiltrate is composed of small lymphocytes with dense basophilic nuclei and a reactive germinal center containing tangible body macrophages. The lymphocytes do not show nuclear atypia accompanied by diffuse plasma cell infiltrate. Occasionally, a granulomatous or eosinophilic component can be seen. Most cases contain B-PSL plasmacytoid dendritic cells with close vicinity to T cells and plasma cells.13
Differential Diagnosis
Several conditions need to be considered in the differential diagnosis, including low-grade cutaneous B-cell lymphoma, nonmycosis fungoides cutaneous T-cell lymphoma, cutaneous metastases, adnexal tumors, polymorphous light eruption, Jessner lymphocytic infiltrate, and tumid lupus. The differential diagnosis includes follicular mycosis fungoides, granulomatous rosacea, cutaneous marginal zone lymphoma, low-grade cutaneous follicular lymphoma, and small- and medium-sized pleomorphic cutaneous T-cell lymphoma. Folliculotropic mycosis fungoides is a cutaneous specific T-cell lymphoma that infiltrates the epidermis in the early stages. Granulomatous rosacea is a chronic inflammatory disease of unknown etiology characterized by facial erythema, transitory or persistent telangiectasias, and often papules and pustules. Low-grade follicular lymphoma is characterized by germinal center lymphoma with at least a pattern of follicular lymphoma. Cutaneous marginal zone lymphoma is a painless post-germinal center B-cell lymphoma, constituted by marginal zone cells, lymphoplasmacytoid cells, and plasma cells. Pseudolymphomatous folliculitis is a rare inflammatory disease classified as lymphomatoid lesions and hypersensitivity syndromes associated with medication.14
Treatment
Treatment options include observation, intralesional or systemic corticosteroids, imiquimod,15 subcutaneous interferon alpha injection,16,17 cryotherapy, surgical excision, and local radiation therapy.18 If offending agents are identified, removal of the agents can lead to curation. When PSL is suspected, all implicated medications should be discontinued. A short-term course of topical or intralesional steroids can be utilized and regression of the lesions may take up to 3 months. If lesions are persistent despite treatment, a careful evaluation for malignant process should be carried out.
Our patient
PSL in a child is a rare entity. A variety of pathologic features can be manifested according to specific agents. For example, insect bites generally cause superficial lymphocytic infiltrations with eosinophils.5,19 In our case, there was no known history of these etiologies, yet a persistent nodular arthropod assault reaction could be considered, particularly in the presence of numerous eosinophils, among other possible reactive causes. The most commonly reported location is the head and neck area.2 Similarly, our patient’s lesion was located on the right frontal scalp. Most cases of PSL consist of a mixture of reactive polytypic B cells, T cells, and macrophages, as well as dendritic cells.3 Our case demonstrated a mixture of B and T cells in IHC.
To treat our patient, we administered 10 mg of intralesional triamcinolone acetonide and followed up after 6 weeks. He did not show improvement with intralesional treatment. The patient then underwent a surgical excision and had another follow up 2 weeks post-excision
Conclusion
PSL is an inflammatory response to various etiologies, including infections, inflammation, and tattoos. Some of the cases are not associated with a preceding factor. Clinically, it appears as pinkcolored, subcutaneous nodules in the skin. PSL of the scalp associated with alopecia has been reported in only a few cases in the literature. The combination of cytokine alteration in affected tissue along with accumulation of B cells in the area may contribute to various etiologies of hair loss. In cases of alopecia with underlying induration and pink discoloration of the scalp, PSL should be kept in mind as a differential diagnosis and prompt clinicians to obtain a biopsy to diagnose this rare entity
Dr Sapmaz is a physician graduated from Marmara University Faculty of Medicine in Istanbul, Turkey. Dr Farabi is a dermatology resident at New York Medical College/Metropolitan Hospital Center in New York, NY. Dr Khachemoune is the Derm DX section editor, and a dermatologist affiliated with SUNY Downstate and VA New York Harbor CareBrooklyn in Brooklyn, NY.
Disclosure: The authors report no relevant financial relationships
References
1. Mehta V, Balachandran C, Hameed S. Lymphocytoma cutis following excision arthroplasty. Indian J Dermatol. 2011;56(1):104-106. doi:10.4103/0019-5154.77569
2. Saltzstein SL. Pulmonary malignant lymphomas and pseudolymphomas: classification, therapy, and prognosis. Cancer. 1963;16:928-955. doi:10.1002/1097- 0142(196307)16:73.0.co;2-h
3. Choi ME, Lee KH, Lim DJ, et al. Clinical and histopathological characteristics of cutaneous lymphoid hyperplasia: a comparative study according to causative factors. J Clin Med. 2020;9(4):1217. doi:10.3390/jcm9041217
4. Brodell RT, Santa Cruz DJ. Cutaneous pseudolymphomas. Dermatol Clin. 1985;3(4):719-734.
5. Caro WA, Helwig HB. Cutaneous lymphoid hyperplasia. Cancer. 1969;24(3):487- 502. doi:10.1002/1097-0142(196909)24:33.0.co;2-7
6. Prabhu V, Shivani A, Pawar VR. Idiopathic cutaneous pseudolymphoma: an enigma. Indian Dermatol Online J. 2014;5(2):224-226. doi:10.4103/2229-5178.131143
7. Castelli E, Caputo V, Morello V, Tomasino RM. Local reactions to tick bites. Am J Dermatopathol. 2008;30(3):241-248. doi:10.1097/DAD.0b013e3181676b60
8. Taguchi R, Sugawara K, Tsuruta D. Case of pseudolymphoma with bald patches on the scalp. J Dermatol. 2015;42(6):654-655. doi:10.1111/1346-8138.12867
9. Cohen I, Levy E, Schreiber H. Alopecia neoplastica due to breast carcinoma. Arch Dermatol. 1961;84:490-492. doi:10.1001/archderm.1961.01580150136023
10. Kim JH, Kim MJ, Sim WY, Lew BL. Alopecia neoplastica due to gastric adenocarcinoma metastasis to the scalp, presenting as alopecia: a case report and literature review. Ann Dermatol. 2014;26(5):624-627. doi:10.5021/ad.2014.26.5.624
11. Garg S, Mishra S, Tondon R, Tripathi K. Hodgkin’s lymphoma presenting as alopecia. Int J Trichology. 2012;4(3):169-171. doi:10.4103/0974-7753.100085
12. Klein AW, Rudolph RI, Leyden JJ. Telogen effluvium as a sign of Hodgkin disease. Arch Dermatol. 1973;108(5):702-703.
13. Mitteldorf C, Kempf W. Cutaneous pseudolymphoma-A review on the spectrum and a proposal for a new classification. J Cutan Pathol. 2020;47(1):76-97. doi:10.1111/cup.13532
14. Mendoza Ramírez JB, Ayala D, Heald A, Moreno GYC. Differential diagnoses of pseudolymphomatous folliculitis: considerations as regards one case. BMJ Case Rep. 2021;14(4):e238291. doi:10.1136/bcr-2020-238291
15. Baumgartner-Nielsen J, Lorentzen H. Imiquimod 5%: a successful treatment for pseudolymphoma. Acta Derm Venereol. 2014;94(4):469. doi:10.2340/00015555-1730
16. Singletary HL, Selim MA, Olsen E. Subcutaneous interferon alfa for the treatment of cutaneous pseudolymphoma. Arch Dermatol. 2012;148(5):572-574. doi:10.1001/archdermatol.2011.1016.
17. Zeng SH, Chen SY, Tang XY, Wang L. A case of cutaneous pseudolymphoma with a distinctive appearance treated successfully by intralesional interferon alpha-1b and corticosteroids. Dermatol Ther. 2020;33(3):e13410. doi:10.1111/dth.13410
18. Joseph D, Irukulla MM, Ahmed SF, Valiyaveettil D, Akram S. Radiotherapy in aggressive cutaneous pseudolymphoma: a case report and review of literature. Radiat Oncol J. 2016;34(1):76-80. doi:10.3857/roj.2016.34.1.76
19. Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25(2):233-244, vii. doi:10.1016/j.det.2007.01.008