Type 2 Inflammation in Atopic Dermatitis
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of The Dermatologist or HMP Global, their employees, and affiliates.

As a clinician and clinical trialist, I live and breathe inflammatory skin disease, with atopic dermatitis (AD) being by far one of the most common conditions I see on a daily basis. When I was in medical school, I was told AD is a disease of childhood, occurs in the flexures, children grow out of it, and that was the end of the story. However, in the year 2025, we recognize AD to be so much more heterogeneous than this, with varying lesional distribution, morphologies, disease duration, and ages of onset. So, to better understand the complexity of AD, let’s dive into the role of type 2 inflammation and how it fuels the signs and symptoms of AD that our patients struggle with every day.
Immune System Basics
To understand type 2 inflammation in AD, we need to review some immune system basics. The immune system consists of the innate immune system and the adaptive immune system. The innate immune system is the body's first line of defense against pathogens, as well as outside organisms, and it produces a rapid, nonspecific response to these outsiders. The main immune cells involved in this innate response are innate lymphoid cells, macrophages, natural killer cells, mast cells, basophils, eosinophils, and neutrophils. On the other hand, the adaptive immune system launches a more targeted reaction against specific pathogens, and it is responsible for immunologic memory. The immune cells that are involved in the adaptive immune system are Th1, Th2, Th17 cells. We are all familiar with these in dermatology.
When we think of inflammation as a whole, there are 3 types of immune responses: type 1, 2 and 3. Each of these responses has an innate component that sounds the alarm, so to speak, initiating the inflammatory cascade against anything and everything, and an adaptive component that is more specific. I know when I hear the word inflammation, I sometimes think of it in the context of disease, but remember inflammation is not inherently bad; it does serve a normal function in our body. Type 1 inflammation has evolved for protection against intracellular pathogens like bacterial viruses. Type 2 inflammation evolved to protect against parasites and noxious environmental substances. And, finally, type 3 inflammation evolved to protect against extracellular bacteria and fungi.
Type 2 inflammation is the allergic arm of this 3-arm immune response, and it is present at all barrier sites, not just in the skin. You can see it in the lungs; you can even see it in the gut. And its ultimate goal is to keep parasites, mites, and worms out of our body. The innate immune system responders involved in type 2 inflammation are basophils, eosinophils, innate lymphoid cells, and mast cells. The adaptive immune system responders are Th2 cells. And, lastly, the key cytokines that are responsible for the cross-talk between all these cells are IL-4, IL-5, IL-13, and IL-31. When everything is functioning normally, this is a great system to protect our body against parasites. But when there is immune dysregulation, we end up with conditions like AD, prurigo nodularis, asthma, eosinophilic esophagitis, and so much more—conditions that have aberrant type 2 inflammation at the core of their pathology.
References:
Howell MD, Kuo FI, Smith PA. Targeting the Janus kinase family in autoimmune skin diseases. Front Immunol. 2019:10:2342. doi:10.3389/fimmu.2019.02342
Leung DYM, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134(4):769-779. doi:10.1016/j.jaci.2014.08.008
Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140(3):633-643. doi:10.1016/j.jaci.2017.07.006
A Closer Look at Type 2 Inflammation
Now, let’s delve into AD and how type 2 inflammation plays a role in its development. This is a condition I treat every single day, and it is an area of disease that is of particular interest to me from a research standpoint. AD is complex: It has a chronic inflammatory piece in the skin, but it also has a systemic component that we need to keep in mind. When we think about the behavioral aspect of AD like scratching, it makes evolutionary sense: If a parasite were trying to penetrate the skin, the body’s instinctive response would be to scratch it off and protect the barrier. But once the skin barrier is damaged by recurrent scratching, the keratinocytes are angry and they sound an alarm that releases TSLP (thymic stromal lymphopoietin), IL-33, and IL-25, and these drive immune cell recruitment and inflammation.
Innate lymphoid cells like dendritic cells present antigens from the outsider to the naive T cell and this begins the T-cell activation process. With the help of IL-4, naive T cells can now differentiate into Th2 cells, which then release IL-4, IL-13, and IL-31. These cytokines can impact the nerves and cause itch, go back to the skin and further disrupt the barrier, and then, through a vicious positive feedback loop, we get more immune cell recruitment and more T-cell differentiation and amplification, with the ultimate result being immune dysregulation and persistence of the inflammation. For simplicity's sake, this is a linear description of what is happening; in reality, everything is happening all at once in our body, with many players activating different parts of the system at any one time.
When the immune system becomes dysregulated—either by overreacting to the wrong trigger or failing to shut off after activation—diseases like AD begin to emerge and manifest clinically. In AD, elevated levels of cytokines, such as IL-4, IL-13, and IL-31, shift from being protective to becoming pathogenic. This causes a decrease in key proteins and lipids that are needed for the structural integrity of the skin barrier, resulting in increased permeability and barrier disruption. We see a decrease in antimicrobial peptides that leads to an increase in Staphylococcus aureus colonization and, ultimately, microbiome dysbiosis ensues. The inflammatory milieu stimulates fibroblasts to overproduce collagen as well, contributing to fibrosis and lichenification. We also see a direct impact on sensory neurons that results in the hallmark symptom of itch. And, finally, we have further increases in pro-inflammatory chemokines and cytokines, which cause the inflammation to persist. Together, these interconnected mechanisms give rise to the clinical picture of AD that we know so well.
Targeting the Pathway
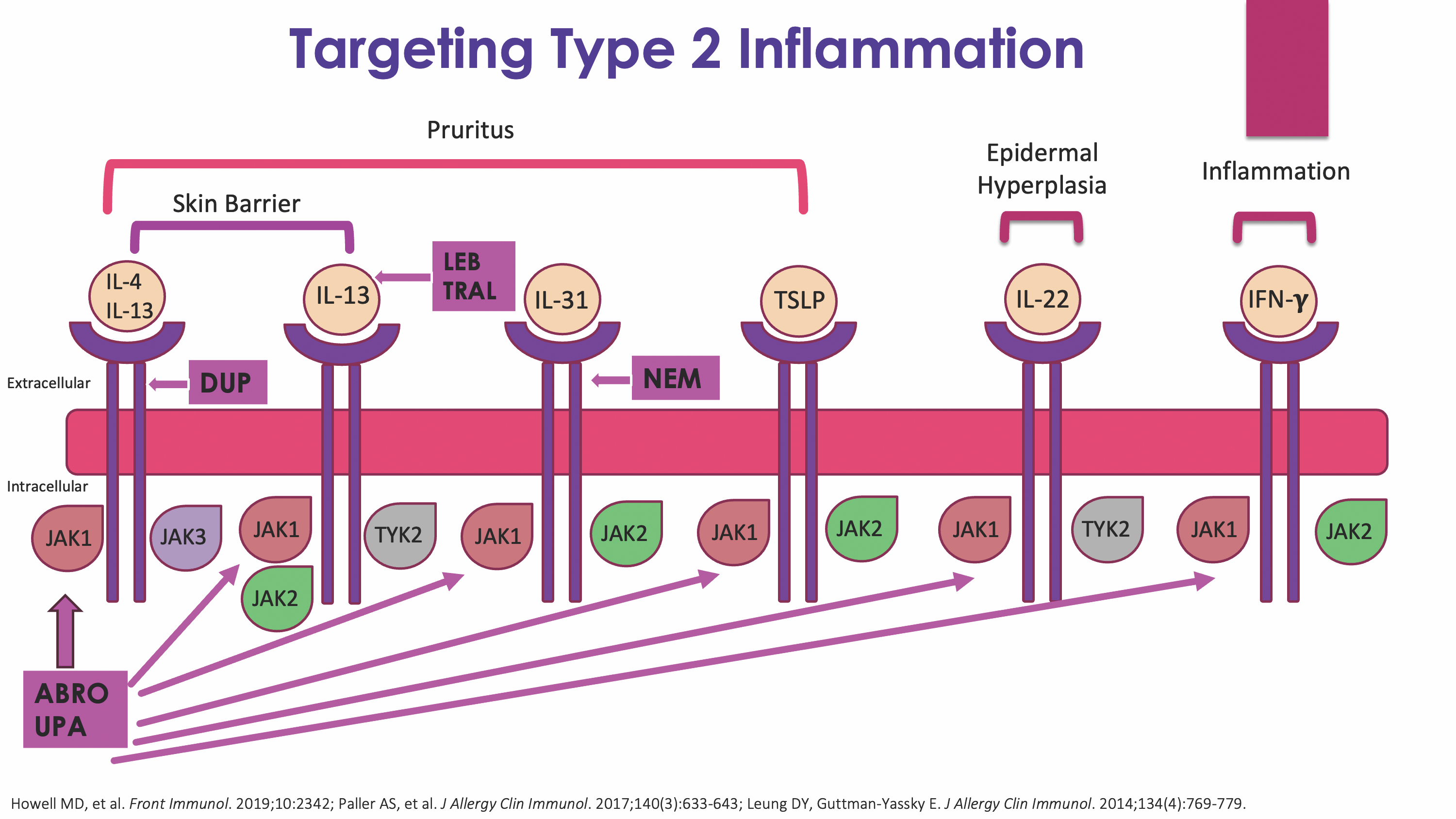
It is no surprise that drug development for AD over the last decade has focused on targeting various aspects of the type 2 inflammatory pathway. Let's review how some of our different therapeutics target the signaling of key cytokines in this pathway (Figure). Dupilumab works by targeting both IL-4 and IL-13 signaling by blocking their shared receptor. Lebrikizumab and tralokinumab work by targeting the IL-13 cytokine, specifically. Nemolizumab works by targeting the IL-31 receptor. Finally, upadacitinib and abrocitinib work by selectively targeting Janus kinase (JAK) 1, which is an intracellular target that impacts the activity of multiple cytokines using the JAK-signal transducer and activator of transcription, or JAK-STAT, pathway to signal.
One thing to note, there is a difference between the selectivity of a JAK inhibitor and the specificity of a biologic. And this is something you want to keep in mind when you are thinking about drug selection for your patients. Biologics have more of a lock-in-key mechanism, with virtually no impact on other pathways because most keys will not work in the wrong lock. In contrast, JAK inhibitors are selective and compete with adenosine triphosphate (ATP) to bind to their preferred site for which they have the highest affinity. For example, a JAK1 selective inhibitor such as abrocitinib likes to bind to the ATP binding domain of JAK1. However, as intracellular concentrations of the drug increase, abrocitinib is likely to bind to the ATP binding domains of other members of the JAK family as well, such as JAK2 and JAK3. This is a so-called spillover effect. And this is why as we pump up the dose of our JAK inhibitors for higher efficacy, we tend to see more adverse reactions.
Conclusion
Not all type 2 inflammation is harmful—in fact, it plays an essential role in protecting our body from parasites and environmental threats. But when this system becomes dysregulated, it drives chronic disease. In AD, cytokines like IL-4, IL-13, and IL-31 are no longer allies—they become central culprits in disrupting the skin barrier, fueling itch, and sustaining inflammation. Fortunately, our expanding therapeutic arsenal now allows us to precisely target these cytokines and their downstream effects. In 2025, managing AD is no longer just about controlling symptoms—it is about intervening in the inflammatory cascade itself and restoring balance to the immune system.