Treatment of Phantom and Residual Limb Pain in Amputees With Targeted Muscle Reinnervation
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Background: Many amputees are left with chronic localized pain, centralized pain, and phantom limb pain or sensation, often resulting from neuromas in the residual limb. Historically, there is no reliably effective intervention for pain associated with neuroma-related residual or phantom limb pain. Targeted muscle reinnervation (TMR) is a surgical procedure first described in 2002 that involves the transfer of residual nerves from amputated limbs to new muscle targets. TMR has been shown to significantly reduce neuroma pain and facilitate the use of prostheses.
Methods: A prospective study was conducted of 61 patients who underwent TMR for neuroma treatment or prevention between 2017 and 2022. Primary outcomes included overall, phantom, and residual limb pain recorded using the Visual Analog Scale (VAS), as well as Patient-Reported Outcomes Measurement Information System (PROMIS) forms for Pain Intensity, Quality, Interference, and Behavior. Retrospective data was collected for a propensity-matched cohort of non-TMR amputees to compare pain outcomes.
Results: TMR was performed for 25 upper extremity and 35 lower extremity amputations, and 5 patients underwent TMR on multiple limbs. Significant reductions were observed in overall limb pain (-3.2 points), phantom limb pain (-2.6 points), and residual limb pain (-3.0 points) for the TMR cohort. Mean PROMIS scores for TMR patients were 49.7 for Pain Intensity, 54.0 for Pain Quality, 55.3 for Pain Interference, and 56.1 for Pain Behavior. At the 8.4-month follow-up, 43.8% of TMR patients (vs 84% of controls) remained on neuromodulators, opioids, or both, for pain control.
Conclusions: TMR improved phantom and residual limb pain in amputees, as evidenced by clinically and statistically significant reductions in pain with reduced need for long-term opioids and/or neuromodulators. These findings support the current understanding of TMR but underscore the need for continued investigation to comprehensively assess the potential of this promising technique in improving the functional outcomes and quality of life in the amputee population.
Introduction
There are an estimated 2 million amputees living in the United States, many of whom suffer from chronic pain that causes measurable impairment in both prosthetic function and quality of life.1,2 This pain is either localized in the residual limb (RLP) or sensed in the removed limb as phantom limb pain (PLP). Neuropathic pain following limb amputation has been associated with both central and peripheral dysfunctions of neural circuitry.3-7 Rates of RLP have been reported from 10% to 76% and are often thought to be the result of symptomatic neuromas.8-12 PLP and phantom limb sensation (PLS) are considered to be the result of incongruent signaling between motor intention and motor or sensory feedback.13 Historical approaches to PLP and RLP have included pharmacological, interventional, and behavioral methods.5,7,14-17 However, these approaches, such as the mirror box therapy, regional analgesia, transcutaneous electrical nerve stimulation, and neuromodulators, have been largely ineffective.18-23
Current surgical efforts involve burying the transected nerve in muscle, bone, or vein, as well as end-to-end nerve coaptation.24-28 While these techniques have shown some efficacy, they lack a terminal nerve receptor for disorganized regenerating axons and thus fail to address the pathology that underlies neuroma formation.13 More recently, products such as nerve caps and conduits have been employed to limit unstructured proliferation of neuromas; however, clinical data is limited, and these still do not supply the injured nerve with an active distal target.29-33 Because of the shortcomings of historical therapeutic approaches, amputee pain management continues to rely heavily on opioids.34
In response to the lack of a standard and effective treatment for PLP and RLP, Dumanian et al developed a technique called targeted muscle reinnervation (TMR).35 TMR creates a denervated muscle segment by dividing its intact motor nerve, then reinnervates the muscle via coaptation of an amputated major mixed nerve with the divided motor nerve.36,37 Unlike other surgical treatments, TMR gives the amputated nerves “somewhere to go and something to do”, allowing for potential reinnervation and more anatomical healing.38
The additional synaptic input to reinnervated muscles may also enhance intuitive control of robotic prostheses.39 In animal models, post-TMR connections have demonstrated nerve-to-nerve healing as well as synaptic input similar to normally innervated muscle on electromyography (EMG).13 In a clinical study of 5 transhumeral amputees, TMR patients had improved prosthesis control with motor control similar to healthy controls, as well as reduced pain.40 Functional magnetic resonance imaging (fMRI) data has shown that amputees who have undergone TMR have cortical maps that more closely resemble uninjured controls than non-TMR amputees, suggesting that efferent and afferent signaling produced by TMR helps to resolve cortical incongruence associated with phantom pain.3,13
Given the promising literature on clinical outcomes of TMR and the mechanisms underlying its utility,41,42 the present study was designed to further evaluate the efficacy of this technique. We hypothesized that TMR will significantly reduce overall pain, PLP, and RLP scores while improving functional outcomes and reducing the need for pharmacologic pain control.
Methods and Materials
Institutional review board approval
A Health Insurance Portability and Accountability Act waiver of authorization was approved by the Texas Tech University Health Sciences Center Lubbock/Odessa Institutional Review Board (IRB #: L21-006).
TMR cohort
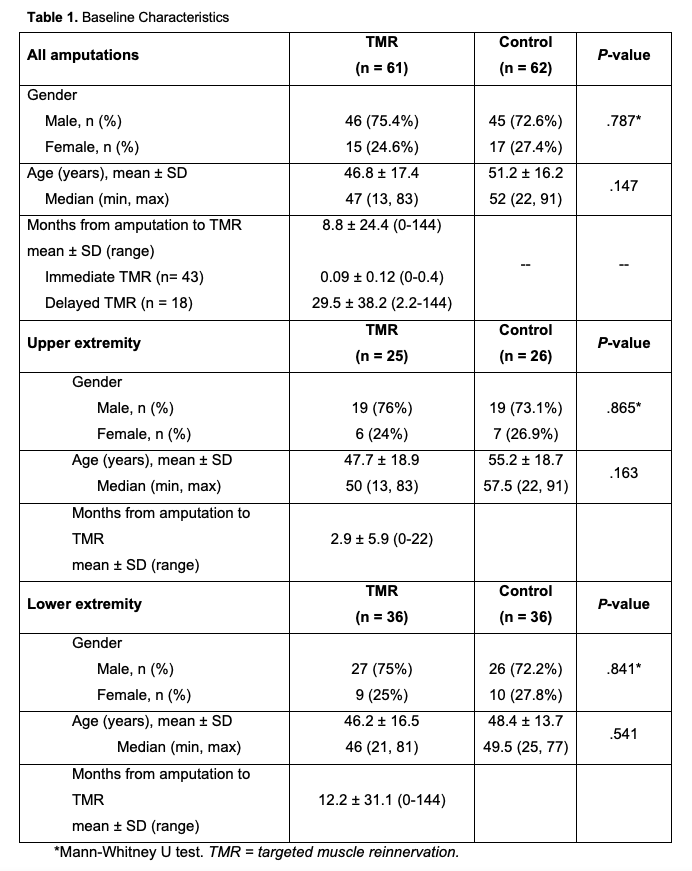
The study period was between 2017 and 2022. Prior to the TMR procedure, demographic and injury data were collected, including age, gender, extremity amputated, and preoperative PLP and/or RLP when possible (Table 1). Pain was measured using a standard 0-to-10-point Visual Analogue Scale (VAS). At each follow-up visit, pain scores were recorded as overall pain, PLP, and RLP. Patient-Reported Outcomes Measurement Information System (PROMIS) forms for Pain Intensity (Short form 3a), Quality (Short form 5a), Interference (Short form 8a), and Behavior (Short form 7a) were also collected when possible43,44 (Table 2).
General amputee population
We benchmarked RLP and PLP among a population of amputees who had not undergone TMR using a standard 0-to-10 Visual Analogue Scale (VAS). These patients were then propensity matched by age, gender, upper extremity (UE) vs lower extremity (LE) amputation, and time to most recent follow-up for comparison with cohort patients (Table 1).
Statistical analysis
The Kolmogorov-Smirnov test of normality was used to determine whether data for each variable was normally distributed. Unpaired t tests were used to compare normally distributed data and Mann-Whitney U tests were used for data that were not normally distributed (Tables 1 and 2).
Results
Demographics
The TMR cohort included 61 patients with an average age of 46.8 years, and 75.4% were male. TMR patients included 59% LE and 41% UE amputations. The average time between initial amputation and TMR was 8.8 months. Forty-three patients underwent immediate TMR (within 2 weeks), and 18 had delayed TMR (mean delay: 29.5 months) (Table 1).
The control group included 62 patients with an average age of 51.2 years, and 2.6% were male. The group included 58% LE amputations and 42% UE amputations. A full breakdown of demographic information and limb involvement for TMR patients and controls is shown in Table 1.
Pain characteristics
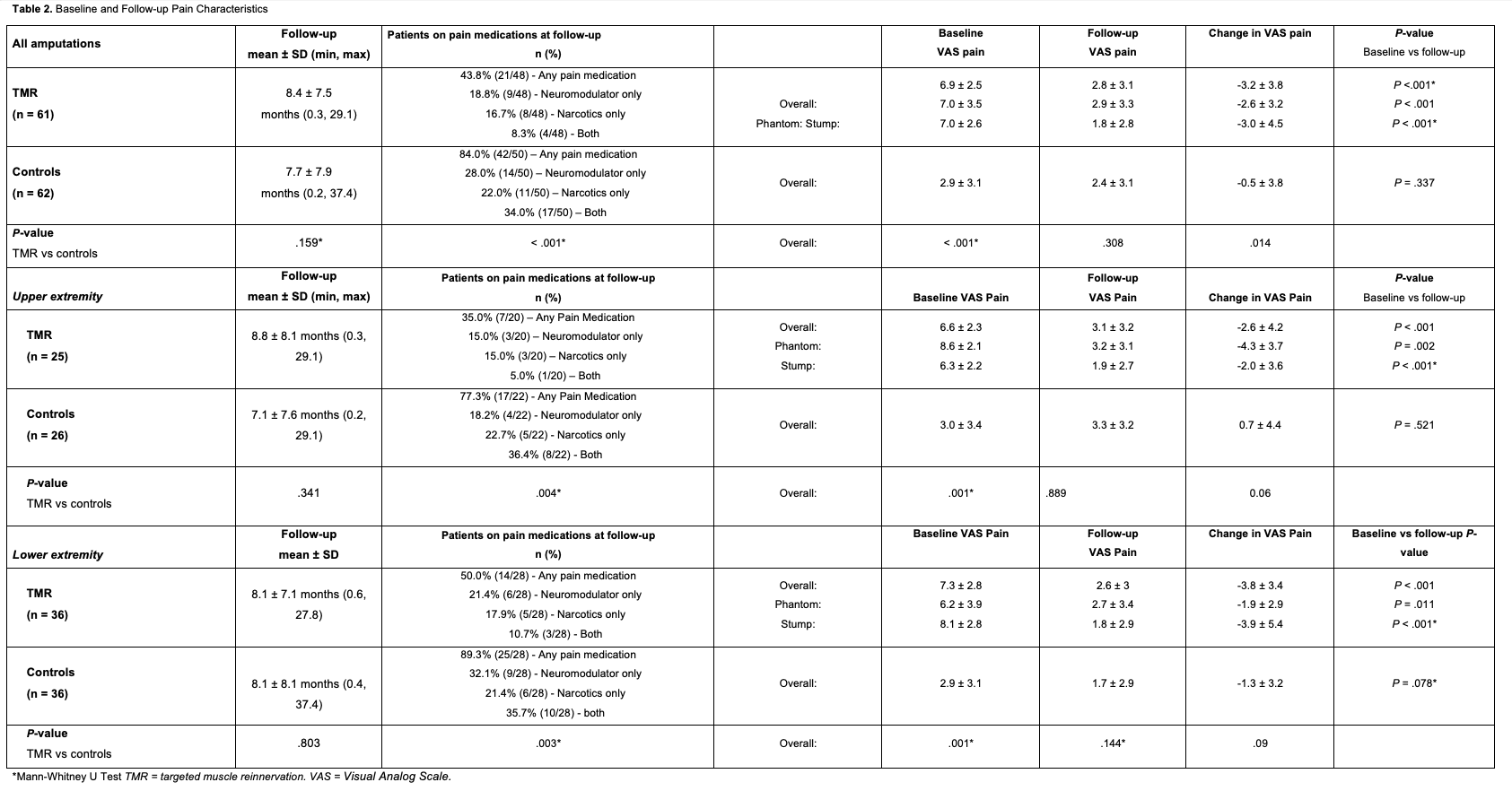
The mean baseline overall pain score for the TMR cohort was 6.9 (controls: 2.9), the mean baseline PLP score was 7.0, and the mean baseline RLP score was 7.0 (Table 2). At the most recent follow-up (mean: 8.4 months, range: 0.3-29.1 months), the mean VAS scores for overall pain, PLP, and RLP were 2.8, 2.9, and 1.8, respectively. Significant reductions were observed in overall pain (-3.2 points, P < .001), PLP (-2.6 points, P < .001), and RLP (-3.0 points, P < .001) for the entire TMR cohort, as well as UE and LE subgroups (Table 2). Mean PROMIS scores for the TMR group were 49.7 for Pain Intensity, 54 for Pain Quality, 55.3 for Pain Interference, and 56.1 for Pain Behavior (Table 3, Figure). At their most recent visit, 43.8% of the TMR patients remained on pain medication (18.8% neuromodulators, 16.7% opioids, and 8.3% both) (Table 2). In the control group, 84% of the patients were still on pain medication at their most recent follow-up (mean: 7.7 months). This included 28% on neuromodulators, 22% on opioids, and 24% on both (Table 2). The average overall VAS pain score (2.4) at the final follow-up was not significantly reduced from baseline (-0.5 points, P = .337). When divided into UE and LE subgroups, neither showed significant reductions in overall pain (Table 2).
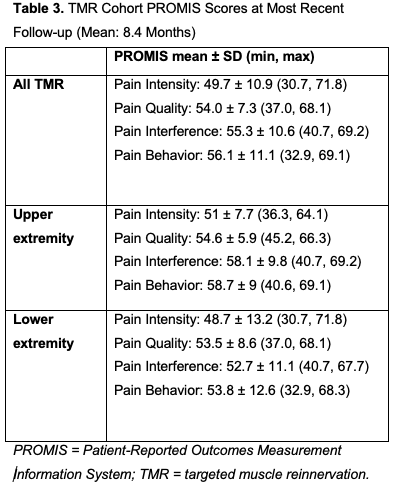
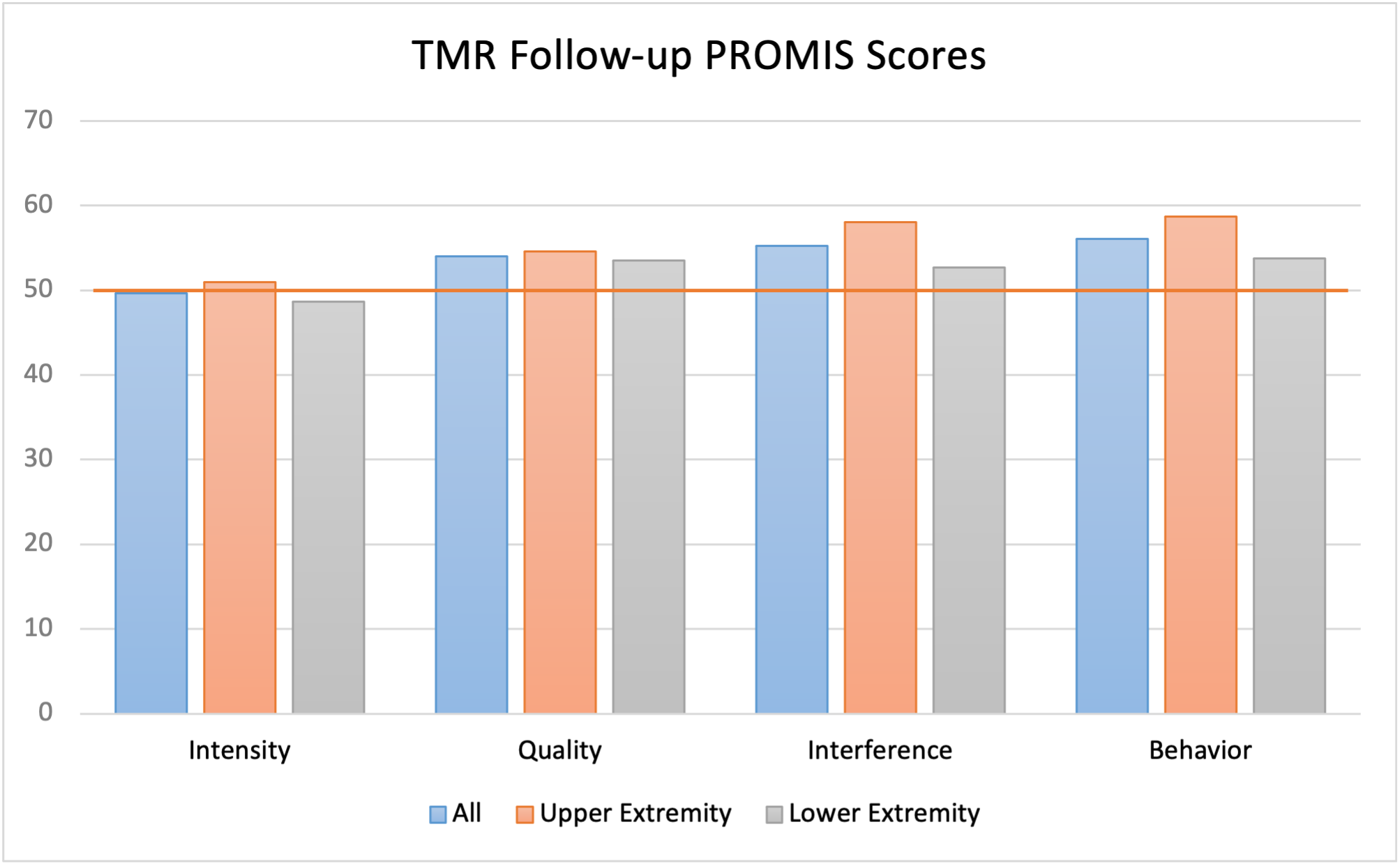
Figure. TMR cohort PROMIS Scores at most recent follow-up (mean: 8.1 months). The horizontal red line at 50 denotes the average pain across the entire US population. PROMIS = Patient-Reported Outcomes Measurement Information System; TMR = targeted muscle reinnervation.
Discussion
Our results indicate that TMR can effectively treat neuropathic pain in amputees, as evidenced by reductions in VAS scores of overall pain, PLP, and RLP in addition to improved PROMIS scores. Reductions in VAS pain greater than 2.0 points are considered to be clinically meaningful.45 Our TMR cohort had an average reduction of 3.2 points in overall pain, 2.6 points in PLP, and 3.0 points in RLP.
A PROMIS score of 50 represents the average pain across the entire US population (both healthy and injured/diseased), with higher scores indicating worse pain. PROMIS scores are scaled such that 1 SD from average is measured by an increment of 10. At follow-up, the TMR cohort’s PROMIS Intensity, Quality, Interference, and Behavior scores were 49.7, 54, 55.3, and 56.1, respectively—all within 1 SD of the baseline pain for the US population as a whole (Figure).
The TMR cohort required fewer pain medications on average compared with controls. Only 43.8% of the TMR patients remained on any pain medications, ranging from Tylenol to opioids such as hydrocodone, at the most recent follow-up, compared with 84% for the control group. Despite having lower initial pain scores, the control group had a higher percentage of patients on neuromodulators and/or opioids at the most recent follow-up (P < .001). Amputees, particularly those with PLP, are at risk of persistent post-amputation opioid use regardless of pre-amputation opioid exposure.34 In addition to the risk of developing an opioid use disorder, both opioids and neuromodulators (eg, gabapentin) may have side effects including nausea, somnolence, dizziness, edema, negative mood changes, and gait disturbance.46 Pain improvements achieved via TMR may reduce the likelihood of long-term use of opioids and/or neuromodulators.
Given the utility of TMR in improving neuropathic pain and the known central changes that occur with prolonged nervous deficiency, surgeons have considered the effects of timing on long-term outcomes with this technique.13,35,47 Pet et al evaluated palpation-induced pain (ie, residual limb hypersensitivity) in primary TMR (average: 4 days post-amputation) and secondary TMR (average: 80 months post-amputation).47 Results showed that 92% of primary TMR patients were free of palpation-induced neuroma pain compared with 87% of secondary TMR patients at a 22-month follow-up.47 In 2019, 2 landmark studies demonstrated the efficacy of TMR in reducing PLP and RLP.13,35 Valerio et al assessed the impact of immediate TMR and showed marked improvements in multiple dimensions of pain, particularly PLP.13 Other studies have confirmed these promising results with immediate TMR for both PLP and RLP; however, direct comparisons between studies are difficult in this population.48-50 In our study, overall pain at follow-up was the only variable that showed significant superiority in the immediate TMR group vs delayed TMR (overall VAS pain: 2.2 vs 4.4; P = .023) (Table 4). However, the sample sizes of these subgroups are too small to effectively parse differences between immediate and delayed TMR within our cohort.
While we are not the first to discuss pain outcomes following TMR, our cohort of 61 patients represents a valuable addition to the existing literature, as most studies are more limited in size.35,48,49,51 Given the high variability of circumstances surrounding amputation, we did not perform statistical analyses directly comparing our cohort with other studies showing improved outcomes with TMR.48-50 However, our results are similar to the findings of a randomized controlled trial conducted by Dumanian et al in 2019, which showed significant improvements from baseline PLP and RLP, as well as superior outcomes when compared with non-TMR controls.35
Regenerative Peripheral Nerve Interface (RPNI) is another surgical technique designed to manage neuroma pain and improve outcomes for patients with nerve injuries or amputations. RPNI involves implanting a transected peripheral nerve into an autologous skeletal muscle graft, which provides a target for nerve regeneration and helps prevent neuroma formation. TMR, on the other hand, involves redirecting severed nerves to new muscle targets to improve prosthetic control and prevent neuroma pain. A direct comparison study by Senger et al demonstrated that both RPNI and TMR significantly reduced pain scores and showed neuromuscular junction reinnervation in a rodent model.52 Additionally, a systematic review by Mauch et al found that both techniques improved neuroma pain in 75% to 100% of patients and PLP in 45% to 80% of patients, with similar efficacy in pain reduction.53
While both techniques are effective, the choice between RPNI and TMR may depend on specific patient factors, surgical expertise, and the desired outcomes, such as improved prosthetic control or pain management. Therefore, RPNI can be considered a viable alternative to TMR for managing neuroma pain and improving patient outcomes.
Limitations
Our study is limited by the requirement of patients to self-assess and report pain outcomes. While the VAS and PROMIS measures have been validated in various clinical contexts, it is important to note that both approaches offer only momentary snapshots of a patient's pain throughout the recovery process.44 The retrospective control group was limited by the extent of pain documentation at routine follow-up visits. Patients were propensity matched based on the level of amputation, mechanism of injury, and demographic characteristics to maximize the value of comparisons. Additionally, it could not be determined whether neuroma pain was linked to prosthetic use because of insufficient soft tissue nerve coverage or merely the presence of a neuroma. It is plausible that RLP could be a result of conditions other than neuroma that are not addressed by this surgical procedure. Lastly, our analysis of neuromodulator and opioid use assumes that the patients were taking these medications as prescribed and had no other source of prescription pain medications.
Conclusions
The results of our study support the growing body of literature that demonstrates improvement of PLP and RLP symptoms in amputees treated with TMR, without complications or revisions related to the procedure. In our cohort, TMR resulted in clinically and statistically significant reductions in pain with a reduced need for long-term opioids and/or neuromodulators. Given the high prevalence of PLP and RLP, TMR has the potential to positively impact functional outcomes and quality of life in our growing amputee population.
Acknowledgments
Authors: Cameron Cox, BA1; Andrew Chen, BS1,2; Gracie Baum, BA1,2; Andrew F. Ibrahim, BS1,2; Evan Hernandez, BS1; Brendan MacKay, MD1
Affiliations: 1Department of Orthopaedic Surgery, Texas Tech University Health Sciences Center, Lubbock, Texas; 2School of Medicine, Texas Tech University Health Sciences Center, Lubbock, Texas
Correspondence: Brendan J. MacKay, MD, Department of Orthopaedic Surgery, Texas Tech University Health Sciences Center, 808 Joliet Avenue, Suite 310, Lubbock, TX 79415, USA, E-mail: brendan.j.mackay@ttuhsc.edu
Ethics: This manuscript was exempt from formal review by the institutional review board (IRB #: L21-006) and documentation can be provided by the corresponding author upon request.
Sources of Support: No funding was received for this work.
Disclosures: The authors disclose no financial or nonfinancial conflicts of interest.
References
1. Pierce RO, Jr, Kernek CB, Ambrose TA II. The plight of the traumatic amputee. Orthopedics. 1993;16(7):793-797. doi:10.3928/0147-7447-19930701-08
2. Tintle SM, Baechler MF, Nanos GP, Forsberg JA, Potter BK. Reoperations following combat-related upper-extremity amputations. J Bone Joint Surg Am. 2012;94(16):e1191-e1196. doi:10.2106/JBJS.K.00197
3. Serino A, Akselrod M, Salomon R, et al. Upper limb cortical maps in amputees with targeted muscle and sensory reinnervation. Brain. 2017;140(11):2993-3011. doi:10.1093/brain/awx242
4. Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7(11):873-881. doi:10.1038/nrn1991.
5. Preissler S, Feiler J, Dietrich C, Hofmann GO, Miltner WHR, Weiss T. Gray matter changes following limb amputation with high and low intensities of phantom limb pain. Cereb Cortex. 2013;23(5):1038-1048. doi:10.1093/cercor/bhs063
6. Vaso A, Adahan H-M, Gjika A, et al. Peripheral nervous system origin of phantom limb pain. Pain. 2014;155(7):1384-1391. doi:10.1016/j.pain.2014.04.018
7. Collins KL, Russell HG, Schumacher PJ, et al. A review of current theories and treatments for phantom limb pain. J Clin Invest. 2018;128(6):2168-2176. doi:10.1172/JCI94003
8. Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehabil. Oct 2005;86(10):1910-1919. doi:10.1016/j.apmr.2005.03.031
9. Ehde DM, Czerniecki JM, Smith DG, et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000;81(8):1039-1044. doi:10.1053/apmr.2000.7583
10. Smith DG, Ehde DM, Legro MW, Reiber GE, del Aguila M, Boone DA. Phantom limb, residual limb, and back pain after lower extremity amputations. Clin Orthop Relat Res. 1999;(361):29-38. doi:10.1097/00003086-199904000-00005
11. Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121-136. doi:10.2147/jpr.S32299
12. Buchheit T, Van de Ven T, Hsia HL, et al. Pain phenotypes and associated clinical risk factors following traumatic amputation: results from Veterans Integrated Pain Evaluation Research (VIPER). Pain Med. 2016;17(1):149-161. doi:10.1111/pme.12848
13. Valerio IL, Dumanian GA, Jordan SW, et al. Preemptive treatment of phantom and residual limb pain with targeted muscle reinnervation at the time of major limb amputation. J Am Coll Surg. 2019;228(3):217-226. doi:10.1016/j.jamcollsurg.2018.12.015
14. Flor H, Elbert T, Knecht S, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375(6531):482-484. doi:10.1038/375482a0
15. Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7(11):873-881. doi:10.1038/nrn1991
16. Montoya P, Ritter K, Huse E, et al. The cortical somatotopic map and phantom phenomena in subjects with congenital limb atrophy and traumatic amputees with phantom limb pain. Eur J Neurosci. 1998;10(3):1095-1102. doi:10.1046/j.1460-9568.1998.00122.x
17. Vaso A, Adahan H-M, Gjika A, et al. Peripheral nervous system origin of phantom limb pain. Pain. 2014;155(7):1384-1391. doi:10.1016/j.pain.2014.04.018
18. Alviar MJM, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;10(10):CD006380. doi:10.1002/14651858.CD006380.pub3
19. Bosanquet DC, Glasbey JC, Stimpson A, Williams IM, Twine CP. Systematic review and meta-analysis of the efficacy of perineural local anaesthetic catheters after major lower limb amputation. Eur J Vasc Endovasc Surg. 2015;50(2):241-249. doi:10.1016/j.ejvs.2015.04.030
20. Richardson C, Kulkarni J. A review of the management of phantom limb pain: challenges and solutions. J Pain Res. 2017;10:1861-1870. doi:10.2147/JPR.S124664
21. Flor H, Denke C, Schaefer M, Grusser S. Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet. 2001;357(9270):1763-1764. doi:10.1016/S0140-6736(00)04890-X
22. Johnson MI, Mulvey MR, Bagnall A-M. Transcutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adults. Cochrane Database Syst Rev. 2015;8(8):CD007264. doi:10.1002/14651858.CD007264.pub3
23. Nikolajsen L, Ilkjaer S, Christensen JH, Krøner K, Jensen TS. Randomised trial of epidural bupivacaine and morphine in prevention of stump and phantom pain in lower-limb amputation. Lancet. 1997;350(9088):1353-1357. doi: 10.1016/S0140-6736(97)06315-0
24. Dellon AL, Mackinnon SE, Pestronk A. Implantation of sensory nerve into muscle: preliminary clinical and experimental observations on neuroma formation. Ann Plast Surg. 1984;12(1):30-40. doi:10.1097/00000637-198401000-00006
25. Wood VE, Mudge MK. Treatment of neuromas about a major amputation stump. J Hand Surg Am. 1987;12(2):302-306. doi:10.1016/s0363-5023(87)80297-6
26. Barberá J, Albert-Pampló R. Centrocentral anastomosis of the proximal nerve stump in the treatment of painful amputation neuromas of major nerves. J Neurosurg. 1993;79(3):331-334. doi:10.3171/jns.1993.79.3.0331
27. Koch H, Hubmer M, Welkerling H, Sandner-Kiesling A, Scharnagl E. The treatment of painful neuroma on the lower extremity by resection and nerve stump transplantation into a vein. Foot Ankle Int. 2004;25(7):476-481. doi:10.1177/107110070402500706
28. Vernadakis AJ, Koch H, Mackinnon SE. Management of neuromas. Clin Plast Surg. 2003;30(2):247-268, vii. doi:10.1016/s0094-1298(02)00104-9
29. Tiwari AP, Lokai T, Albin B, Yang IH. A review on the technological advances and future perspectives of axon guidance and regeneration in peripheral nerve repair. Bioengineering (Basel). 2022;9(10):562. doi:10.3390/bioengineering9100562
30. Onode E, Uemura T, Hama S, et al. Nerve-end capping treatment with a polyglycolic acid conduit for rat sciatic neuroma: a preliminary report. J Reconstr Microsurg. 2022;38(9):711-720. doi:10.1055/s-0042-1757208
31. Onode E, Uemura T, Takamatsu K, et al. Nerve capping with a nerve conduit for the treatment of painful neuroma in the rat sciatic nerve. J Neurosurg. 2019;132(3):856-864. doi:10.3171/2018.10.Jns182113
32. Eberlin KR, Ducic I. Surgical algorithm for neuroma management: a changing treatment paradigm. Plast Reconstr Surg Glob Open. 2018;6(10):e1952. doi:10.1097/gox.0000000000001952
33. Yan H, Zhang F, Wang C, Xia Z, Mo X, Fan C. The role of an aligned nanofiber conduit in the management of painful neuromas in rat sciatic nerves. Ann Plast Surg. 2015;74(4):454-461. doi:10.1097/sap.0000000000000266
34. Steen T, Lirk PB, Sigurdsson MI. The demographics of persistent opioid consumption following limb amputation. Acta Anaesthesiol Scand. 2020;64(3):361-367. doi:10.1111/aas.13497
35. Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg. 2019;270(2):238-246. doi:10.1097/SLA.0000000000003088
36. Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004;28(3):245-253. doi:10.3109/03093640409167756
37. Kuiken TA, Miller LA, Lipschutz RD, et al. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet. 2007;369(9559):371-380. doi:10.1016/S0140-6736(07)60193-7
38. Chappell AG, Jordan SW, Dumanian GA. Targeted muscle reinnervation for treatment of neuropathic pain. Clin Plast Surg. 2020;47(2):285-293. doi:10.1016/j.cps.2020.01.002
39. Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA, Dumanian GA. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res. 2014;472(10):2984-2990. doi:10.1007/s11999-014-3528-7
40. Farina D, Castronovo AM, Vujaklija I, et al. Common synaptic input to motor neurons and neural drive to targeted reinnervated muscles. J Neurosci. 2017;37(46):11285-11292. doi:10.1523/JNEUROSCI.1179-17.2017
41. Lotze M, Grodd W, Birbaumer N, Erb M, Huse E, Flor H. Does use of a myoelectric prosthesis prevent cortical reorganization and phantom limb pain? Nat Neurosci. 1999;2(6):501-502. doi:10.1038/9145
42. Preißler S, Thielemann D, Dietrich C, Hofmann GO, Miltner WHR, Weiss T. Preliminary evidence for training-induced changes of morphology and phantom limb pain. Front Hum Neurosci. 2017;11:319. doi:10.3389/fnhum.2017.00319
43. Cella D, Riley W, Stone A, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179-1194. doi:10.1016/j.jclinepi.2010.04.011
44. Askew RL, Cook KF, Revicki DA, Cella D, Amtmann D. Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. J Clin Epidemiol. 2016;73:103-111. doi:10.1016/j.jclinepi.2015.08.035
45. Decrouy-Duruz V, Christen T, Raffoul W. Evaluation of surgical treatment for neuropathic pain from neuroma in patients with injured peripheral nerves. J Neurosurg. 2018;128(4):1235-1240. doi:10.3171/2017.1.Jns161778
46. Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD007938. doi:10.1002/14651858.CD007938.pub4
47. Pet MA, Ko JH, Friedly JL, Mourad PD, Smith DG. Does targeted nerve implantation reduce neuroma pain in amputees? Clin Orthop Relat Res. 2014;472(10):2991-3001. doi:10.1007/s11999-014-3602-1
48. Frantz TL, Everhart JS, West JM, Ly TV, Phieffer LS, Valerio IL. Targeted muscle reinnervation at the time of major limb amputation in traumatic amputees: early experience of an effective treatment strategy to improve pain. JB JS Open Access. 2020;5(2):e0067. doi:10.2106/jbjs.Oa.19.00067
49. Michno DA, Woollard ACS, Kang NV. Clinical outcomes of delayed targeted muscle reinnervation for neuroma pain reduction in longstanding amputees. J Plast Reconstr Aesthet Surg. 2019;72(9):1576-1606. doi:10.1016/j.bjps.2019.05.005
50. McNamara CT, Iorio ML. Targeted muscle reinnervation: outcomes in treating chronic pain secondary to extremity amputation and phantom limb syndrome. J Reconstr Microsurg.2020;36(4):235-240. doi:10.1055/s-0039-1700559
51. Mioton LM, Dumanian GA, Shah N, et al. Targeted muscle reinnervation improves residual limb pain, phantom limb pain, and limb function: a prospective study of 33 major limb amputees. Clin Orthop Relat Res. 2020;478(9):2161-2167. doi:10.1097/corr.0000000000001323
52. Senger JB, Hardy P, Thorkelsson A, et al. A direct comparison of targeted muscle reinnervation and regenerative peripheral nerve interfaces to prevent neuroma pain. Neurosurgery. 2023;93(5):1180-1191. doi:10.1227/neu.0000000000002541
53. Mauch JT, Kao DS, Friedly JL, Liu Y. Targeted muscle reinnervation and regenerative peripheral nerve interfaces for pain prophylaxis and treatment: a systematic review. PM R. 2023;15(11):1457-1465. doi:10.1002/pmrj.12972