A Case of Extrasphincteric Enterocutaneous Fistula After Scrotal Reconstruction for Fournier Gangrene: Use of a Novel Salvage Technique
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of ePlasty or HMP Global, their employees, and affiliates.
Abstract
Anocutaneous and rectocutaneous fistulas are associated with high morbidity and mortality, and complex fistulas often pose challenges to surgical repair. Here, the authors report a case of a rare 7-cm extrasphincteric fistula that extended from the base of the phallus to the external anal canal in a patient with a history of scrotal reconstruction after Fournier gangrene. Despite the anterior location of the cutaneous sinus tract and initial concern for penile urethral involvement, intraoperative instillation of methylene blue revealed a long extrasphincteric anocutaneous fistula. With colorectal surgery co-involvement, the mature tract was dissected free from the urethra, base of the penis, and scrotum, and redirected inferiorly and diverted to a new position anterior to the anal sphincter complex. This technique created a significantly shorter tract that salvaged the scrotal reconstruction, preserved the sphincter complex, and minimized risk of infection to the scrotal reconstruction. This novel technique can be used as either a temporary or permanent solution to complex mature fistulas in high-risk patients.
Introduction
Fournier gangrene is a rare and rapidly progressive form of necrotizing fasciitis affecting the deep and superficial tissues of the perineal, anal, scrotal, and genital areas.1,2 In this condition, prompt and aggressive surgical debridement is critical, as any significant delay increases patient morbidity and mortality.3 Scrotal Fournier gangrene may require multiple debridements and temporizing dressings until the infection is cleared and the wound is stable enough for reconstruction.4 Options for scrotal and perineal reconstruction can be performed using rotational fasciocutaneous flaps, muscle flaps, or simple skin grafting.5 Depending on patient circumstances, surgical intervention often involves a multispecialty approach involving plastic surgery, urology, general surgery, colorectal surgery, and obstetrics and gynecology.6
In small-scale studies, Fournier gangrene has been associated with anocutaneous fistulas, a common anorectal disease involving an abnormal tract connecting the anal canal to the perianal skin.7,8 Anal fistulas are classified in relation to the external sphincter, such as intersphincteric, transsphincteric, suprasphincteric, and extrasphincteric.9 Extrasphincteric anal fistulas are very rare fistulas that typically connect the distal rectum to the perineum via a tract that remains outside the sphincter complex.8,10 With extrasphincteric anal fistulas, surgical treatment is challenging because of the location and the need to preserve the anal sphincter complex, with many therapies placing patients at risk for fecal incontinence and infection.8 Plastic surgery involvement usually occurs when the fistula repair requires the placement of flaps from regional or distant locations. To date, there are few reports of extrasphincteric fistulas in patients with recent Fournier gangrene, and treatment of these fistulas is prone to significant morbidity.11
In this study, we report our experience with a complex extrasphincteric enterocutaneous fistula originating at the anorectal junction in a patient with a history of Fournier gangrene and scrotal reconstruction. The rarity and complexity of this case necessitated the development and application of a novel tract-pedicalization technique for treating the anocutaneous fistula while preserving the sphincteric complex and salvaging the scrotal reconstruction.
Case Presentation
The patient was a 62-year-old man with a past medical history of Fournier gangrene of unknown etiology, status post-debridement and reconstruction of the scrotum using bilateral pudendal artery perforator flaps in September 2022. Within the immediate postoperative period, the patient developed chronic sinus tracts and, 8 months later, received an excision of a blind-ended sinus tract to the left scrotal reconstruction. He also had a persistent sinus tract at the base of the phallus that developed 1 year post-reconstruction, which was managed initially with dressings. Given concern for urethral involvement, a retrograde urethrogram was performed, which did not show any connection to the urethra. However, after failure to resolve, the patient presented in June 2024 for sinus tract exploration by plastic surgery and urology.
Surgical technique
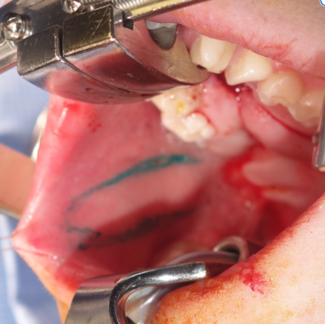
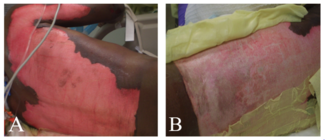
The case began with the injection of methylene blue into the sinus tract at the base of the phallus with a 20G angiocatheter. A lacrimal probe was used to explore the sinus tract, which measured 7 cm in depth. An “S”-shaped incision was created to re-elevate the left pudendal artery flap and expose the sinus tract. The superficial fascia was dissected with careful preservation of the left pudendal artery, which supplied the fasciocutaneous flap. After raising the flap, the tract was noted to be well-formed with significant scarring (Figure 1). The tract was dissected from the bulbospongiosus muscle with no apparent injury to the urethra, bulbospongiosus muscle, or underlying testicular structures. The tract did not appear to lead toward the urethra and directed instead toward the anorectal junction, which led to the exploration of anocutaneous involvement. Methylene blue was used to stain the anterior portion of the anorectal junction, indicating an anorectal cutaneous fistula. Colorectal surgery was consulted intraoperatively.
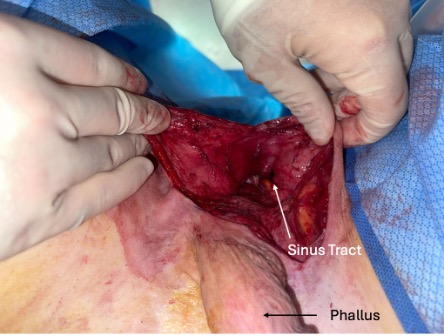
Figure 1. Visible sinus tract following the elevation of the fasciocutaneous flap.
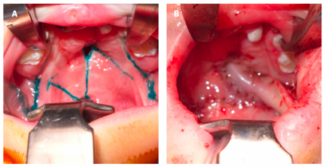
A fistula probe was placed at the external opening of the tract, and a small internal opening within the anterior anal canal (2 cm proximal to the anal verge and to the left of the midline) was observed. Given that this tract was still epithelialized and already dissected to 1 cm proximal to the anal opening, the decision was made to remove the tract from the scrotal area and tunnel it through an area of the perineum adjacent to the anal sphincter complex. An incision was made in the perineal skin and dissected to the opening within the previously exposed field of the perineum. The external opening of the fistula tract was then brought through this newly created tunnel. A 4.1-cm length of the tract was excised and sent to pathology for review. The remaining tract was matured to the perineal skin with simple 3-0 Vicryl sutures (Johnson & Johnson MedTech), allowing the modified fistula tract to drain in a lower risk region (Figure 2).
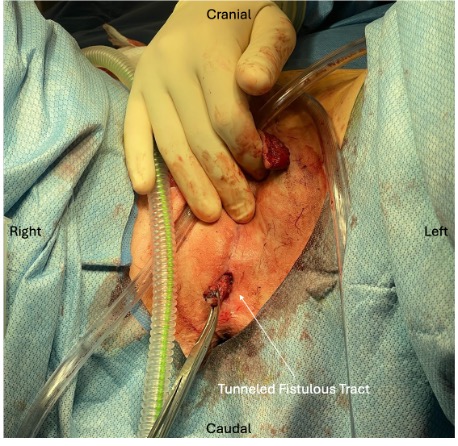
Figure 2. Fistulous tract position after surgical tunneling to the perianal region.
Following inset of the modified fistula tract, the left pudendal artery flap was re-inset. A 10 French Blake drain was placed into the perineum. The wound was closed in layers using 3-0 Monocryl sutures (Johnson & Johnson MedTech) for all layers: interrupted buried simple sutures in the superior fascial system, interrupted deep dermal simple sutures, and running subcuticular sutures. The suture line was dressed with Xeroform.

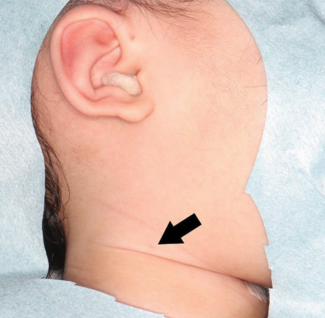
Figure 3. Drainage of the fistula tract at the 5-week postoperative visit.
At the latest follow-up, which occurred 3 months post-reconstruction, the patient remains stable with no evidence of infection or flap failure.
Discussion
Pudendal artery flaps can be excellent options for scrotal and perineal reconstruction because of their versatility and reliability. The pudendal flap is a durable fasciocutaneous flap with a reliable blood supply from the pudendal artery and minimal donor site morbidity.12 Among other benefits, it can safely be re-raised and inset for secondary closures, which is especially important in complicated reconstructions such as those necessitated by Fournier gangrene or when treating ano- or rectocutaneous fistulas that cannot be managed with conservative procedures.
Extrasphincteric ano- and rectocutaneous fistulas are both rare and complex, with clinical courses that are often protracted and require sophisticated interventions and operative procedures.13 Because of their infrequency, diagnosis is often the first challenge in their clinical management. Surgical exploration remains the gold standard for characterization of the fistula tract, including possible involvement of critical structures. However, surgical procedures require individualized planning based on the location of the tract, degree of tissue involvement, and types of tissues involved.13 Often, preoperative imaging with a fistulogram is used in this diagnosis.14 However, in the case of our patient, given the initial presentation of a sinus tract rather than a fistula, a fistulogram was not sought prior to operative exploration.
In this circumstance, we chose a technique that would allow for a shorter tract that could reduce inflammation and secretion at the level of the scrotal reconstruction. After defining the tract, we elected to proceed with excision of the fistulous tract and transposition of the tract to a new site that was closer to the anus. This decreased flap morbidity and infection rates by mitigating communication with enteric tracts and minimizing formation of abscesses, cellulitis, and seromas.15,16 While the pursued treatment may allow for preservation of a scrotal reconstruction, it also does not preclude the use of other permanent surgical treatments in the future.
In the case of this patient’s extrasphincteric fistula, there was no standard or optimal surgical method. For simple fistulas, various treatment options including endorectal flaps, seton bands, or fistulotomies can be considered. In this case, an endorectal flap would have been very difficult, given a high risk of flap retraction or suture rupture.17 Given the length and location of the tract in this patient, a seton band is not typically preferred.18 Finally, a fistulotomy is not optimal when larger portions of the sphincteric muscle are involved in the tract. Ligation of an intersphincteric fistula tract may be a suitable option for patients with anorectal fistulas after scrotal reconstruction;19 this technique involves disconnection of the internal opening from the fistula tract without dividing the anal sphincter complex, therefore preserving continence and leading to high success rates.20
In summary, scrotal reconstruction following Fournier gangrene can be associated with myriad possible complications, and formation of an extrasphincteric ano- or rectocutaneous fistula should remain on the differential diagnosis for any chronic draining wound after this reconstruction. This novel solution for the treatment of extrasphinteric ano- or rectocutaneous fistulas, which includes dissection and inset of a pedicled fistula tract, can be paired with the dissection and re-inset of fasciocutaneous flaps and utilized as a technique to minimize further complications. Additionally, if a more definitive treatment is eventually pursued, this approach stabilizes the fistula tract, allowing time for more informed patient-physician decision-making and preoperative planning.
Acknowledgments
Authors: Micaela J. Tobin, BA1; John B. Park, PharmD1; Tricia Mae Raquepo, BA1; Mohammed Yamin, BS1; Helen Xun, MD1; Adam Jac1; Kristen T. Crowell, MD2; Marissa A. Kent, MD3; Ryan P. Cauley, MD, MPH1
Affiliations: 1Division of Plastic and Reconstructive Surgery, Department of Surgery, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts; 2Division of Colon and Rectal Surgery, Department of Surgery, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts; 3Division of Urology, Department of Surgery, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts
Correspondence: Ryan P. Cauley, MD, MPH, Division of Plastic and Reconstructive Surgery, Department of Surgery, Beth Israel Deaconess Medical Center, Harvard Medical School, LMOB 5A, Boston, MA, 02215, USA. Email: rcauley@bidmc.harvard.edu; Tel: 203-550-0144; ORCID: 0000-0001-8291-433X.
Ethics: Permission was obtained from the patient for publication.
Disclosures: The authors disclose no relevant financial or nonfinancial conflicts of interest.
References
- Yoshino H, Kawakami K, Yoshino G, Sawada K. Case of anal fistula with Fournier's gangrene in an obese type 2 diabetes mellitus patient. J Diabetes Investig. 2016;7(2):276-278. doi:10.1111/jdi.12355
- Leslie SW, Foreman J. Fournier gangrene. 2025 Feb 15. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–.
- Kabay S, Yucel M, Yaylak F, et al. The clinical features of Fournier's gangrene and the predictivity of the Fournier's Gangrene Severity Index on the outcomes. Int Urol Nephrol. 2008;40(4):997-1004. doi:10.1007/s11255-008-9401-4
- Chawla SN, Gallop C, Mydlo JH. Fournier's gangrene: an analysis of repeated surgical debridement. Eur Urol. 2003;43(5):572-575. doi:10.1016/s0302-2838(03)00102-7
- Suda S, Hayashida K. Crafting contours: a comprehensive guide to scrotal reconstruction. Life (Basel). 2024;14(2):223. doi:10.3390/life14020223
- Susini P, Marcaccini G, Efica J, et al. Fournier's gangrene surgical reconstruction: a systematic review. J Clin Med. 2024;13(14):4085. doi:10.3390/jcm13144085
- Kuo CF, Wang WS, Lee CM, Liu CP, Tseng HK. Fournier's gangrene: ten-year experience in a medical center in northern Taiwan. J Microbiol Immunol Infect. 2007;40(6):500-506.
- Carr S, Velasco AL. Fistula-in-ano. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-.
- Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63(1):1-12. doi:10.1002/bjs.1800630102
- Zhao WW, Yu J, Shu J, et al. Precise and comprehensive evaluation of perianal fistulas, classification and related complications using magnetic resonance imaging. Am J Transl Res. 2023;15(5):3674-3685.
- Fasth SB, Nordgren S, Hultén L. Clinical course and management of suprasphincteric and extrasphincteric fistula-in-ano. Acta Chir Scand. 1990;156(5):397-402.
- Insua-Pereira I, Ferreira PC, Teixeira S, Barreiro D, Silva Á. Fournier's gangrene: a review of reconstructive options. Cent European J Urol. 2020;73(1):74-79. doi:10.5173/ceju.2020.0060
- Loungnarath R, Dietz DW, Mutch MG, Birnbaum EH, Kodner IJ, Fleshman JW. Fibrin glue treatment of complex anal fistulas has low success rate. Dis Colon Rectum. 2004;47(4):432-436. doi:10.1007/s10350-003-0076-8
- Ross H. Operative surgery for enterocutaneous fistula. Clin Colon Rectal Surg. 2010;23(3):190-194. doi:10.1055/s-0030-1262987
- Tuma F, Crespi Z, Wolff CJ, Daniel DT, Nassar AK. Enterocutaneous fistula: a simplified clinical approach. Cureus. 2020;12(4):e7789. doi:10.7759/cureus.7789
- Hyman N. Anorectal abscess and fistula. Prim Care. 1999;26(1):69-80. doi:10.1016/s0095-4543(05)70102-0
- Schouten WR, Zimmerman DD, Briel JW. Transanal advancement flap repair of transsphincteric fistulas. Dis Colon Rectum. 1999;42(11):1419-1422; discussion 1422-1423. doi:10.1007/BF02235039
- Cariati A. Fistulotomy or seton in anal fistula: a decisional algorithm. Updates Surg. 2013;65(3):201-205. doi:10.1007/s13304-013-0216-1
- Hong KD, Kang S, Kalaskar S, Wexner SD. Ligation of intersphincteric fistula tract (LIFT) to treat anal fistula: systematic review and meta-analysis. Tech Coloproctol. 2014;18(8):685-691. doi: =10.1007/s10151-014-1183-3
- Rojanasakul A, Pattanaarun J, Sahakitrungruang C, Tantiphlachiva K. Total anal sphincter saving technique for fistula-in-ano; the ligation of intersphincteric fistula tract. J Med Assoc Thai. 2007;90(3):581-586.